| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histamine H1 receptor |
|---|
| Ligand | BDBM50402126 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_884700 (CHEMBL2215148) |
|---|
| Ki | 50.12±n/a nM |
|---|
| Citation |  Furber, M; Alcaraz, L; Luckhurst, C; Bahl, A; Beaton, H; Bowers, K; Collington, J; Denton, R; Donald, D; Kinchin, E; MacDonald, C; Rigby, A; Riley, R; Soars, M; Springthorpe, B; Webborn, P Discovery and evolution of phenoxypiperidine hydroxyamide dual CCR3/H1 antagonists. Part II: optimising in vivo clearance. Bioorg Med Chem Lett22:7707-10 (2012) [PubMed] Article Furber, M; Alcaraz, L; Luckhurst, C; Bahl, A; Beaton, H; Bowers, K; Collington, J; Denton, R; Donald, D; Kinchin, E; MacDonald, C; Rigby, A; Riley, R; Soars, M; Springthorpe, B; Webborn, P Discovery and evolution of phenoxypiperidine hydroxyamide dual CCR3/H1 antagonists. Part II: optimising in vivo clearance. Bioorg Med Chem Lett22:7707-10 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histamine H1 receptor |
|---|
| Name: | Histamine H1 receptor |
|---|
| Synonyms: | H1R | HH1R | HISTAMINE H1 | HRH1 | HRH1_HUMAN |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 55808.72 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Cell pellets from SK-N-MC cells transfected with human H1 receptor were used in binding assay. |
|---|
| Residue: | 487 |
|---|
| Sequence: | MSLPNSSCLLEDKMCEGNKTTMASPQLMPLVVVLSTICLVTVGLNLLVLYAVRSERKLHT
VGNLYIVSLSVADLIVGAVVMPMNILYLLMSKWSLGRPLCLFWLSMDYVASTASIFSVFI
LCIDRYRSVQQPLRYLKYRTKTRASATILGAWFLSFLWVIPILGWNHFMQQTSVRREDKC
ETDFYDVTWFKVMTAIINFYLPTLLMLWFYAKIYKAVRQHCQHRELINRSLPSFSEIKLR
PENPKGDAKKPGKESPWEVLKRKPKDAGGGSVLKSPSQTPKEMKSPVVFSQEDDREVDKL
YCFPLDIVHMQAAAEGSSRDYVAVNRSHGQLKTDEQGLNTHGASEISEDQMLGDSQSFSR
TDSDTTTETAPGKGKLRSGSNTGLDYIKFTWKRLRSHSRQYVSGLHMNRERKAAKQLGFI
MAAFILCWIPYFIFFMVIAFCKNCCNEHLHMFTIWLGYINSTLNPLIYPLCNENFKKTFK
RILHIRS
|
|
|
|---|
| BDBM50402126 |
|---|
| n/a |
|---|
| Name | BDBM50402126 |
|---|
| Synonyms: | CHEMBL2207672 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H29Cl2N3O6S |
|---|
| Mol. Mass. | 582.496 |
|---|
| SMILES | Cc1c(Cl)ccc(OC2CCN(C[C@H](O)CNC(=O)c3c[nH]c(=O)c4cc(ccc34)S(C)(=O)=O)CC2)c1Cl |r| |
|---|
| Structure | 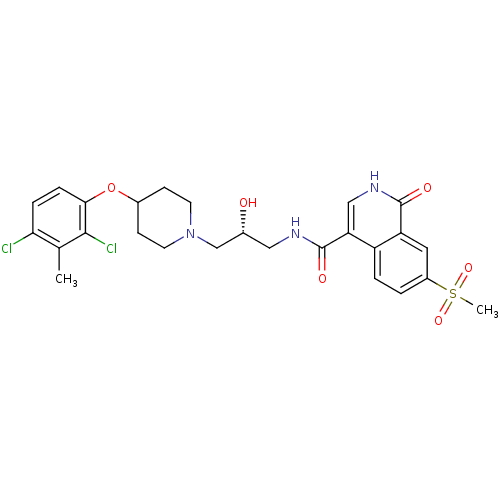
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Furber, M; Alcaraz, L; Luckhurst, C; Bahl, A; Beaton, H; Bowers, K; Collington, J; Denton, R; Donald, D; Kinchin, E; MacDonald, C; Rigby, A; Riley, R; Soars, M; Springthorpe, B; Webborn, P Discovery and evolution of phenoxypiperidine hydroxyamide dual CCR3/H1 antagonists. Part II: optimising in vivo clearance. Bioorg Med Chem Lett22:7707-10 (2012) [PubMed] Article
Furber, M; Alcaraz, L; Luckhurst, C; Bahl, A; Beaton, H; Bowers, K; Collington, J; Denton, R; Donald, D; Kinchin, E; MacDonald, C; Rigby, A; Riley, R; Soars, M; Springthorpe, B; Webborn, P Discovery and evolution of phenoxypiperidine hydroxyamide dual CCR3/H1 antagonists. Part II: optimising in vivo clearance. Bioorg Med Chem Lett22:7707-10 (2012) [PubMed] Article