| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM2261 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_195671 (CHEMBL800744) |
|---|
| IC50 | 1250±n/a nM |
|---|
| Citation |  Hoffman, JM; Smith, AM; Rooney, CS; Fisher, TE; Wai, JS; Thomas, CM; Bamberger, DL; Barnes, JL; Williams, TM; Jones, JH Synthesis and evaluation of 2-pyridinone derivatives as HIV-1-specific reverse transcriptase inhibitors. 4. 3-[2-(Benzoxazol-2-yl)ethyl]-5-ethyl-6-methylpyridin-2(1H)-one and analogues. J Med Chem36:953-66 (1993) [PubMed] Hoffman, JM; Smith, AM; Rooney, CS; Fisher, TE; Wai, JS; Thomas, CM; Bamberger, DL; Barnes, JL; Williams, TM; Jones, JH Synthesis and evaluation of 2-pyridinone derivatives as HIV-1-specific reverse transcriptase inhibitors. 4. 3-[2-(Benzoxazol-2-yl)ethyl]-5-ethyl-6-methylpyridin-2(1H)-one and analogues. J Med Chem36:953-66 (1993) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM2261 |
|---|
| n/a |
|---|
| Name | BDBM2261 |
|---|
| Synonyms: | 2-Pyridinone derivative 41 | 3-[(1,3-benzoxazol-2-ylamino)methyl]-5-ethyl-6-methyl-1,2-dihydropyridin-2-one | 3-{[(Benzoxazol-2-yl)amino]methyl}-5-ethyl-6-methylpyridin-2(1H)-one | CHEMBL175089 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H17N3O2 |
|---|
| Mol. Mass. | 283.3251 |
|---|
| SMILES | CCc1cc(CNc2nc3ccccc3o2)c(=O)[nH]c1C |
|---|
| Structure | 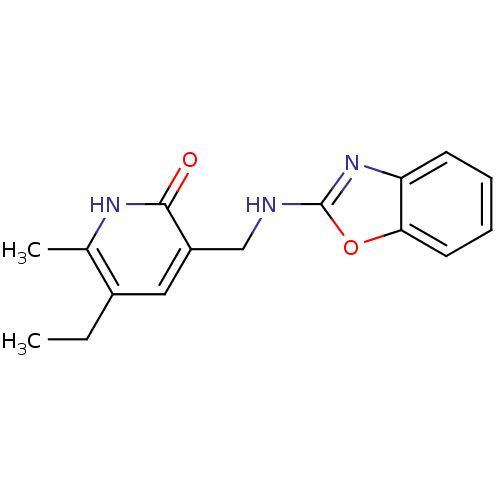
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hoffman, JM; Smith, AM; Rooney, CS; Fisher, TE; Wai, JS; Thomas, CM; Bamberger, DL; Barnes, JL; Williams, TM; Jones, JH Synthesis and evaluation of 2-pyridinone derivatives as HIV-1-specific reverse transcriptase inhibitors. 4. 3-[2-(Benzoxazol-2-yl)ethyl]-5-ethyl-6-methylpyridin-2(1H)-one and analogues. J Med Chem36:953-66 (1993) [PubMed]
Hoffman, JM; Smith, AM; Rooney, CS; Fisher, TE; Wai, JS; Thomas, CM; Bamberger, DL; Barnes, JL; Williams, TM; Jones, JH Synthesis and evaluation of 2-pyridinone derivatives as HIV-1-specific reverse transcriptase inhibitors. 4. 3-[2-(Benzoxazol-2-yl)ethyl]-5-ethyl-6-methylpyridin-2(1H)-one and analogues. J Med Chem36:953-66 (1993) [PubMed]