| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50408241 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1582 (CHEMBL616993) |
|---|
| Ki | 8000±n/a nM |
|---|
| Citation |  Elworthy, TR; Ford, AP; Bantle, GW; Morgans, DJ; Ozer, RS; Palmer, WS; Repke, DB; Romero, M; Sandoval, L; Sjogren, EB; Talamás, FX; Vazquez, A; Wu, H; Arredondo, NF; Blue, DR; DeSousa, A; Gross, LM; Kava, MS; Lesnick, JD; Vimont, RL; Williams, TJ; Zhu, QM; Pfister, JR; Clarke, DE N-arylpiperazinyl-N'-propylamino derivatives of heteroaryl amides as functional uroselective alpha 1-adrenoceptor antagonists. J Med Chem40:2674-87 (1997) [PubMed] Article Elworthy, TR; Ford, AP; Bantle, GW; Morgans, DJ; Ozer, RS; Palmer, WS; Repke, DB; Romero, M; Sandoval, L; Sjogren, EB; Talamás, FX; Vazquez, A; Wu, H; Arredondo, NF; Blue, DR; DeSousa, A; Gross, LM; Kava, MS; Lesnick, JD; Vimont, RL; Williams, TJ; Zhu, QM; Pfister, JR; Clarke, DE N-arylpiperazinyl-N'-propylamino derivatives of heteroaryl amides as functional uroselective alpha 1-adrenoceptor antagonists. J Med Chem40:2674-87 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT1A | 5-hydroxytryptamine receptor 1A | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_MOUSE | Gpcr18 | Htr1a | Serotonin 1a (5-HT1a) receptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46192.09 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | Q64264 |
|---|
| Residue: | 421 |
|---|
| Sequence: | MDMFSLGQGNNTTTSLEPFGTGGNDTGLSNVTFSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSNPNECTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKKGAGT
SFGTSSAPPPKKSLNGQPGSGDCRRSAENRAVGTPCANGAVRQGEDDATLEVIEVHRVGN
SKGHLPLPSESGATSYVPACLERKNERTAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPELLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
R
|
|
|
|---|
| BDBM50408241 |
|---|
| n/a |
|---|
| Name | BDBM50408241 |
|---|
| Synonyms: | CHEMBL89030 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H34N4O2 |
|---|
| Mol. Mass. | 410.5524 |
|---|
| SMILES | COc1ccccc1N1CCN(CCCNc2cc(C)ccc2C(=O)N(C)C)CC1 |
|---|
| Structure | 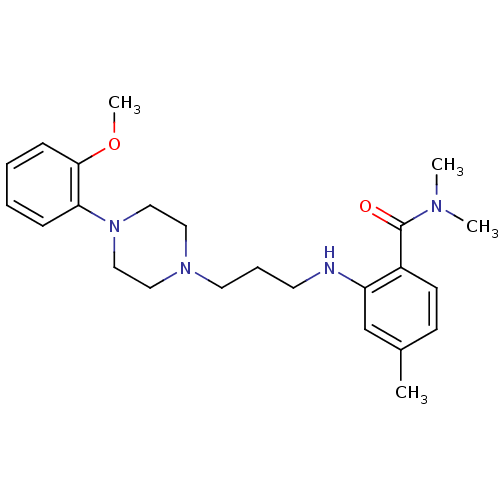
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Elworthy, TR; Ford, AP; Bantle, GW; Morgans, DJ; Ozer, RS; Palmer, WS; Repke, DB; Romero, M; Sandoval, L; Sjogren, EB; Talamás, FX; Vazquez, A; Wu, H; Arredondo, NF; Blue, DR; DeSousa, A; Gross, LM; Kava, MS; Lesnick, JD; Vimont, RL; Williams, TJ; Zhu, QM; Pfister, JR; Clarke, DE N-arylpiperazinyl-N'-propylamino derivatives of heteroaryl amides as functional uroselective alpha 1-adrenoceptor antagonists. J Med Chem40:2674-87 (1997) [PubMed] Article
Elworthy, TR; Ford, AP; Bantle, GW; Morgans, DJ; Ozer, RS; Palmer, WS; Repke, DB; Romero, M; Sandoval, L; Sjogren, EB; Talamás, FX; Vazquez, A; Wu, H; Arredondo, NF; Blue, DR; DeSousa, A; Gross, LM; Kava, MS; Lesnick, JD; Vimont, RL; Williams, TJ; Zhu, QM; Pfister, JR; Clarke, DE N-arylpiperazinyl-N'-propylamino derivatives of heteroaryl amides as functional uroselective alpha 1-adrenoceptor antagonists. J Med Chem40:2674-87 (1997) [PubMed] Article