| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Hepatocyte growth factor receptor |
|---|
| Ligand | BDBM50427138 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_940625 (CHEMBL2327567) |
|---|
| Kd | 10±n/a nM |
|---|
| Citation |  Northrup, AB; Katcher, MH; Altman, MD; Chenard, M; Daniels, MH; Deshmukh, SV; Falcone, D; Guerin, DJ; Hatch, H; Li, C; Lu, W; Lutterbach, B; Allison, TJ; Patel, SB; Reilly, JF; Reutershan, M; Rickert, KW; Rosenstein, C; Soisson, SM; Szewczak, AA; Walker, D; Wilson, K; Young, JR; Pan, BS; Dinsmore, CJ Discovery of 1-[3-(1-methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo[4,5]cyclohepta[1,2-b]pyridin-7-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide (MK-8033): A Specific c-Met/Ron dual kinase inhibitor with preferential affinity for the activated state of c-Met. J Med Chem56:2294-310 (2013) [PubMed] Article Northrup, AB; Katcher, MH; Altman, MD; Chenard, M; Daniels, MH; Deshmukh, SV; Falcone, D; Guerin, DJ; Hatch, H; Li, C; Lu, W; Lutterbach, B; Allison, TJ; Patel, SB; Reilly, JF; Reutershan, M; Rickert, KW; Rosenstein, C; Soisson, SM; Szewczak, AA; Walker, D; Wilson, K; Young, JR; Pan, BS; Dinsmore, CJ Discovery of 1-[3-(1-methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo[4,5]cyclohepta[1,2-b]pyridin-7-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide (MK-8033): A Specific c-Met/Ron dual kinase inhibitor with preferential affinity for the activated state of c-Met. J Med Chem56:2294-310 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Hepatocyte growth factor receptor |
|---|
| Name: | Hepatocyte growth factor receptor |
|---|
| Synonyms: | Hepatocyte growth factor receptor | Hepatocyte growth factor receptor (MET) | Hepatocyte growth factor receptor (c-MET) | Hepatocyte growth factor receptor (cMET) | MET | MET_HUMAN | Met proto-oncogene (hepatocyte growth factor receptor) | Proto-oncogene c-Met | Tyrosine-protein kinase Met (c-Met) | Tyrosine-protein kinase Met (cMet) | c-Met kinase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 155559.73 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08581 |
|---|
| Residue: | 1390 |
|---|
| Sequence: | MKAPAVLAPGILVLLFTLVQRSNGECKEALAKSEMNVNMKYQLPNFTAETPIQNVILHEH
HIFLGATNYIYVLNEEDLQKVAEYKTGPVLEHPDCFPCQDCSSKANLSGGVWKDNINMAL
VVDTYYDDQLISCGSVNRGTCQRHVFPHNHTADIQSEVHCIFSPQIEEPSQCPDCVVSAL
GAKVLSSVKDRFINFFVGNTINSSYFPDHPLHSISVRRLKETKDGFMFLTDQSYIDVLPE
FRDSYPIKYVHAFESNNFIYFLTVQRETLDAQTFHTRIIRFCSINSGLHSYMEMPLECIL
TEKRKKRSTKKEVFNILQAAYVSKPGAQLARQIGASLNDDILFGVFAQSKPDSAEPMDRS
AMCAFPIKYVNDFFNKIVNKNNVRCLQHFYGPNHEHCFNRTLLRNSSGCEARRDEYRTEF
TTALQRVDLFMGQFSEVLLTSISTFIKGDLTIANLGTSEGRFMQVVVSRSGPSTPHVNFL
LDSHPVSPEVIVEHTLNQNGYTLVITGKKITKIPLNGLGCRHFQSCSQCLSAPPFVQCGW
CHDKCVRSEECLSGTWTQQICLPAIYKVFPNSAPLEGGTRLTICGWDFGFRRNNKFDLKK
TRVLLGNESCTLTLSESTMNTLKCTVGPAMNKHFNMSIIISNGHGTTQYSTFSYVDPVIT
SISPKYGPMAGGTLLTLTGNYLNSGNSRHISIGGKTCTLKSVSNSILECYTPAQTISTEF
AVKLKIDLANRETSIFSYREDPIVYEIHPTKSFISGGSTITGVGKNLNSVSVPRMVINVH
EAGRNFTVACQHRSNSEIICCTTPSLQQLNLQLPLKTKAFFMLDGILSKYFDLIYVHNPV
FKPFEKPVMISMGNENVLEIKGNDIDPEAVKGEVLKVGNKSCENIHLHSEAVLCTVPNDL
LKLNSELNIEWKQAISSTVLGKVIVQPDQNFTGLIAGVVSISTALLLLLGFFLWLKKRKQ
IKDLGSELVRYDARVHTPHLDRLVSARSVSPTTEMVSNESVDYRATFPEDQFPNSSQNGS
CRQVQYPLTDMSPILTSGDSDISSPLLQNTVHIDLSALNPELVQAVQHVVIGPSSLIVHF
NEVIGRGHFGCVYHGTLLDNDGKKIHCAVKSLNRITDIGEVSQFLTEGIIMKDFSHPNVL
SLLGICLRSEGSPLVVLPYMKHGDLRNFIRNETHNPTVKDLIGFGLQVAKGMKYLASKKF
VHRDLAARNCMLDEKFTVKVADFGLARDMYDKEYYSVHNKTGAKLPVKWMALESLQTQKF
TTKSDVWSFGVLLWELMTRGAPPYPDVNTFDITVYLLQGRRLLQPEYCPDPLYEVMLKCW
HPKAEMRPSFSELVSRISAIFSTFIGEHYVHVNATYVNVKCVAPYPSLLSSEDNADDEVD
TRPASFWETS
|
|
|
|---|
| BDBM50427138 |
|---|
| n/a |
|---|
| Name | BDBM50427138 |
|---|
| Synonyms: | CHEMBL2323775 | MK-8033 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H21N5O3S |
|---|
| Mol. Mass. | 471.531 |
|---|
| SMILES | Cn1cc(cn1)-c1cnc2ccc3ccc(CS(=O)(=O)NCc4ccccn4)cc3c(=O)c2c1 |
|---|
| Structure | 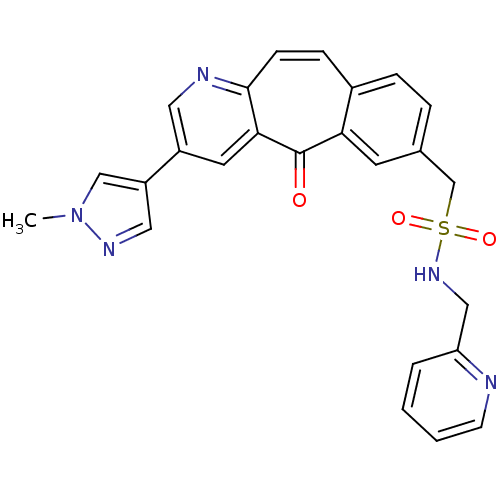
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Northrup, AB; Katcher, MH; Altman, MD; Chenard, M; Daniels, MH; Deshmukh, SV; Falcone, D; Guerin, DJ; Hatch, H; Li, C; Lu, W; Lutterbach, B; Allison, TJ; Patel, SB; Reilly, JF; Reutershan, M; Rickert, KW; Rosenstein, C; Soisson, SM; Szewczak, AA; Walker, D; Wilson, K; Young, JR; Pan, BS; Dinsmore, CJ Discovery of 1-[3-(1-methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo[4,5]cyclohepta[1,2-b]pyridin-7-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide (MK-8033): A Specific c-Met/Ron dual kinase inhibitor with preferential affinity for the activated state of c-Met. J Med Chem56:2294-310 (2013) [PubMed] Article
Northrup, AB; Katcher, MH; Altman, MD; Chenard, M; Daniels, MH; Deshmukh, SV; Falcone, D; Guerin, DJ; Hatch, H; Li, C; Lu, W; Lutterbach, B; Allison, TJ; Patel, SB; Reilly, JF; Reutershan, M; Rickert, KW; Rosenstein, C; Soisson, SM; Szewczak, AA; Walker, D; Wilson, K; Young, JR; Pan, BS; Dinsmore, CJ Discovery of 1-[3-(1-methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo[4,5]cyclohepta[1,2-b]pyridin-7-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide (MK-8033): A Specific c-Met/Ron dual kinase inhibitor with preferential affinity for the activated state of c-Met. J Med Chem56:2294-310 (2013) [PubMed] Article