| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin G/H synthase 2 |
|---|
| Ligand | BDBM50427623 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_941815 (CHEMBL2329806) |
|---|
| IC50 | 90±n/a nM |
|---|
| Citation |  Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin G/H synthase 2 |
|---|
| Name: | Prostaglandin G/H synthase 2 |
|---|
| Synonyms: | Cox-2 | Cox2 | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2) | Glucocorticoid-regulated inflammatory cyclooxygenase | Gripghs | Macrophage activation-associated marker protein P71/73 | PES-2 | PGH synthase 2 | PGH2_MOUSE | PGHS-2 | PHS II | Pghs-b | Prostaglandin G/H synthase (cyclooxygenase) | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2 | Ptgs2 | TIS10 protein | Tis10 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 69020.39 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | Q05769 |
|---|
| Residue: | 604 |
|---|
| Sequence: | MLFRAVLLCAALGLSQAANPCCSNPCQNRGECMSTGFDQYKCDCTRTGFYGENCTTPEFL
TRIKLLLKPTPNTVHYILTHFKGVWNIVNNIPFLRSLIMKYVLTSRSYLIDSPPTYNVHY
GYKSWEAFSNLSYYTRALPPVADDCPTPMGVKGNKELPDSKEVLEKVLLRREFIPDPQGS
NMMFAFFAQHFTHQFFKTDHKRGPGFTRGLGHGVDLNHIYGETLDRQHKLRLFKDGKLKY
QVIGGEVYPPTVKDTQVEMIYPPHIPENLQFAVGQEVFGLVPGLMMYATIWLREHNRVCD
ILKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQ
NRIASEFNTLYHWHPLLPDTFNIEDQEYSFKQFLYNNSILLEHGLTQFVESFTRQIAGRV
AGGRNVPIAVQAVAKASIDQSREMKYQSLNEYRKRFSLKPYTSFEELTGEKEMAAELKAL
YSDIDVMELYPALLVEKPRPDAIFGETMVELGAPFSLKGLMGNPICSPQYWKPSTFGGEV
GFKIINTASIQSLICNNVKGCPFTSFNVQDPQPTKTATINASASHSRLDDINPTVLIKRR
STEL
|
|
|
|---|
| BDBM50427623 |
|---|
| n/a |
|---|
| Name | BDBM50427623 |
|---|
| Synonyms: | CHEMBL2323476 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H13ClFNO3 |
|---|
| Mol. Mass. | 345.752 |
|---|
| SMILES | Cc1c(CC(O)=O)c2cc(F)ccc2n1C(=O)c1ccc(Cl)cc1 |
|---|
| Structure | 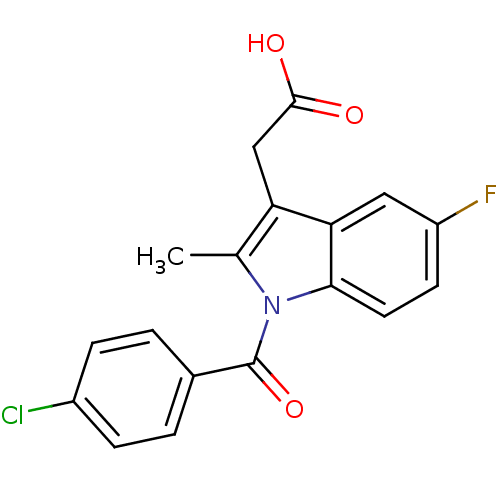
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article
Liedtke, AJ; Adeniji, AO; Chen, M; Byrns, MC; Jin, Y; Christianson, DW; Marnett, LJ; Penning, TM Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (Type 5 17ß-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J Med Chem56:2429-46 (2013) [PubMed] Article