| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Enoyl-acyl carrier reductase ENR |
|---|
| Ligand | BDBM50431920 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_953658 (CHEMBL2352562) |
|---|
| IC50 | 58±n/a nM |
|---|
| Citation |  Cheng, G; Muench, SP; Zhou, Y; Afanador, GA; Mui, EJ; Fomovska, A; Lai, BS; Prigge, ST; Woods, S; Roberts, CW; Hickman, MR; Lee, PJ; Leed, SE; Auschwitz, JM; Rice, DW; McLeod, R Design, synthesis, and biological activity of diaryl ether inhibitors of Toxoplasma gondii enoyl reductase. Bioorg Med Chem Lett23:2035-43 (2013) [PubMed] Article Cheng, G; Muench, SP; Zhou, Y; Afanador, GA; Mui, EJ; Fomovska, A; Lai, BS; Prigge, ST; Woods, S; Roberts, CW; Hickman, MR; Lee, PJ; Leed, SE; Auschwitz, JM; Rice, DW; McLeod, R Design, synthesis, and biological activity of diaryl ether inhibitors of Toxoplasma gondii enoyl reductase. Bioorg Med Chem Lett23:2035-43 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Enoyl-acyl carrier reductase ENR |
|---|
| Name: | Enoyl-acyl carrier reductase ENR |
|---|
| Synonyms: | Enoyl-acyl carrier reductase | Enoyl-acyl carrier reductase (ENR) | Enoyl-acyl carrier reductase, putative |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 43663.67 |
|---|
| Organism: | Toxoplasma gondii |
|---|
| Description: | ChEMBL_1290812 |
|---|
| Residue: | 417 |
|---|
| Sequence: | MVGFKLLTLGAFVAGELTLVGPAGTMAFTVPNATGAKPLVTSVSVRPSWSSARQNAFSSS
SSRSQSSVRPHSAFVTNRLETAGETGTQHRAADSAAGVGAAQSAFPIDLRGQTAFVAGVA
DSHGYGWAIAKHLASAGARVALGTWPPVLGLFQKSLQSGRLDEDRKLPDGSLIEFAGVYP
LDAAFDKPEDVPQDIKDNKRYAGVDGYTIKEVAVKVKQDLGNIDILVHSLANGPEVTKPL
LETSRKGYLAASSNSAYSFVSLLQHFGPIMNEGGSAVTLSYLAAERVVPGYGGGMSSAKA
ALESDTRTLAWEAGQKYGVRVNAISAGPLKSRAASAIGKSGEKSFIDYAIDYSYNNAPLR
RDLHSDDVGGAALFLLSPLARAVSGVTLYVDNGLHAMGQAVDSRSMPPLQRATQEIN
|
|
|
|---|
| BDBM50431920 |
|---|
| n/a |
|---|
| Name | BDBM50431920 |
|---|
| Synonyms: | 5‐(4‐chloro‐2‐hydroxyphenoxy)‐N‐(5‐methyl‐1,2‐ oxazol‐3‐yl)furan‐2‐carboxamide (32) | CHEMBL2347835 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H11ClN2O5 |
|---|
| Mol. Mass. | 334.711 |
|---|
| SMILES | Cc1cc(NC(=O)c2ccc(Oc3ccc(Cl)cc3O)o2)no1 |
|---|
| Structure | 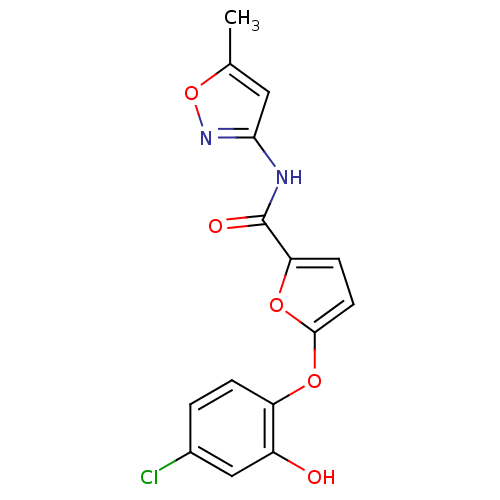
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cheng, G; Muench, SP; Zhou, Y; Afanador, GA; Mui, EJ; Fomovska, A; Lai, BS; Prigge, ST; Woods, S; Roberts, CW; Hickman, MR; Lee, PJ; Leed, SE; Auschwitz, JM; Rice, DW; McLeod, R Design, synthesis, and biological activity of diaryl ether inhibitors of Toxoplasma gondii enoyl reductase. Bioorg Med Chem Lett23:2035-43 (2013) [PubMed] Article
Cheng, G; Muench, SP; Zhou, Y; Afanador, GA; Mui, EJ; Fomovska, A; Lai, BS; Prigge, ST; Woods, S; Roberts, CW; Hickman, MR; Lee, PJ; Leed, SE; Auschwitz, JM; Rice, DW; McLeod, R Design, synthesis, and biological activity of diaryl ether inhibitors of Toxoplasma gondii enoyl reductase. Bioorg Med Chem Lett23:2035-43 (2013) [PubMed] Article