| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50432235 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_950952 (CHEMBL2350278) |
|---|
| IC50 | 1500±n/a nM |
|---|
| Citation |  Amblard, F; Zhang, H; Zhou, L; Shi, J; Bobeck, DR; Nettles, JH; Chavre, S; McBrayer, TR; Tharnish, P; Whitaker, T; Coats, SJ; Schinazi, RF Synthesis and evaluation of non-dimeric HCV NS5A inhibitors. Bioorg Med Chem Lett23:2031-4 (2013) [PubMed] Article Amblard, F; Zhang, H; Zhou, L; Shi, J; Bobeck, DR; Nettles, JH; Chavre, S; McBrayer, TR; Tharnish, P; Whitaker, T; Coats, SJ; Schinazi, RF Synthesis and evaluation of non-dimeric HCV NS5A inhibitors. Bioorg Med Chem Lett23:2031-4 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50432235 |
|---|
| n/a |
|---|
| Name | BDBM50432235 |
|---|
| Synonyms: | CHEMBL2347323 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H24BrN7O3 |
|---|
| Mol. Mass. | 490.354 |
|---|
| SMILES | COC(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1c1nc(c(Br)[nH]1)-c1ccc(cc1)N=[N+]=[N-] |r| |
|---|
| Structure | 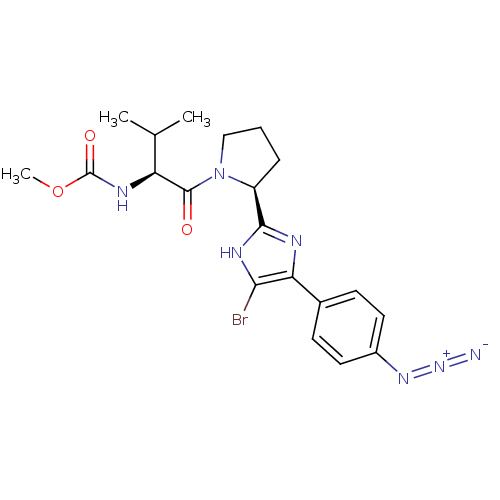
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Amblard, F; Zhang, H; Zhou, L; Shi, J; Bobeck, DR; Nettles, JH; Chavre, S; McBrayer, TR; Tharnish, P; Whitaker, T; Coats, SJ; Schinazi, RF Synthesis and evaluation of non-dimeric HCV NS5A inhibitors. Bioorg Med Chem Lett23:2031-4 (2013) [PubMed] Article
Amblard, F; Zhang, H; Zhou, L; Shi, J; Bobeck, DR; Nettles, JH; Chavre, S; McBrayer, TR; Tharnish, P; Whitaker, T; Coats, SJ; Schinazi, RF Synthesis and evaluation of non-dimeric HCV NS5A inhibitors. Bioorg Med Chem Lett23:2031-4 (2013) [PubMed] Article