| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Calcitonin gene-related peptide type 1 receptor |
|---|
| Ligand | BDBM50356282 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_969057 (CHEMBL2400791) |
|---|
| Ki | 0.021000±n/a nM |
|---|
| Citation |  Chaturvedula, PV; Mercer, SE; Pin, SS; Thalody, G; Xu, C; Conway, CM; Keavy, D; Signor, L; Cantor, GH; Mathias, N; Moench, P; Denton, R; Macci, R; Schartman, R; Whiterock, V; Davis, C; Macor, JE; Dubowchik, GM Discovery of (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (BMS-742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett23:3157-61 (2013) [PubMed] Article Chaturvedula, PV; Mercer, SE; Pin, SS; Thalody, G; Xu, C; Conway, CM; Keavy, D; Signor, L; Cantor, GH; Mathias, N; Moench, P; Denton, R; Macci, R; Schartman, R; Whiterock, V; Davis, C; Macor, JE; Dubowchik, GM Discovery of (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (BMS-742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett23:3157-61 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Calcitonin gene-related peptide type 1 receptor |
|---|
| Name: | Calcitonin gene-related peptide type 1 receptor |
|---|
| Synonyms: | Adrenomedullin receptor AM1; CALCRL/RAMP2 | CALCRL | CALRL_HUMAN | CGRP type 1 receptor | CGRP type 1 receptor mRNA | CGRPR | Calcitonin gene-related peptide (CGRP) receptor | Calcitonin gene-related peptide 1 (CGRP) | Calcitonin receptor-like receptor | Calcitonin receptor-like receptor (CLR) | Human CL receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 52980.45 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q16602 |
|---|
| Residue: | 461 |
|---|
| Sequence: | MEKKCTLYFLVLLPFFMILVTAELEESPEDSIQLGVTRNKIMTAQYECYQKIMQDPIQQA
EGVYCNRTWDGWLCWNDVAAGTESMQLCPDYFQDFDPSEKVTKICDQDGNWFRHPASNRT
WTNYTQCNVNTHEKVKTALNLFYLTIIGHGLSIASLLISLGIFFYFKSLSCQRITLHKNL
FFSFVCNSVVTIIHLTAVANNQALVATNPVSCKVSQFIHLYLMGCNYFWMLCEGIYLHTL
IVVAVFAEKQHLMWYYFLGWGFPLIPACIHAIARSLYYNDNCWISSDTHLLYIIHGPICA
ALLVNLFFLLNIVRVLITKLKVTHQAESNLYMKAVRATLILVPLLGIEFVLIPWRPEGKI
AEEVYDYIMHILMHFQGLLVSTIFCFFNGEVQAILRRNWNQYKIQFGNSFSNSEALRSAS
YTVSTISDGPGYSHDCPSEHLNGKSIHDIENVLLKPENLYN
|
|
|
|---|
| BDBM50356282 |
|---|
| n/a |
|---|
| Name | BDBM50356282 |
|---|
| Synonyms: | CHEMBL1910936 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H29F2N5O3 |
|---|
| Mol. Mass. | 557.5905 |
|---|
| SMILES | Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| |
|---|
| Structure | 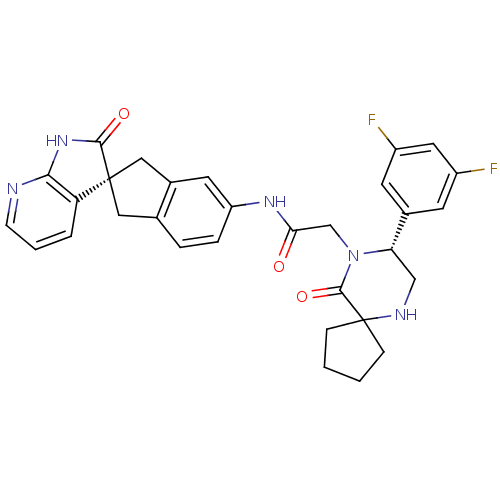
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chaturvedula, PV; Mercer, SE; Pin, SS; Thalody, G; Xu, C; Conway, CM; Keavy, D; Signor, L; Cantor, GH; Mathias, N; Moench, P; Denton, R; Macci, R; Schartman, R; Whiterock, V; Davis, C; Macor, JE; Dubowchik, GM Discovery of (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (BMS-742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett23:3157-61 (2013) [PubMed] Article
Chaturvedula, PV; Mercer, SE; Pin, SS; Thalody, G; Xu, C; Conway, CM; Keavy, D; Signor, L; Cantor, GH; Mathias, N; Moench, P; Denton, R; Macci, R; Schartman, R; Whiterock, V; Davis, C; Macor, JE; Dubowchik, GM Discovery of (R)-N-(3-(7-methyl-1H-indazol-5-yl)-1-(4-(1-methylpiperidin-4-yl)-1-oxopropan-2-yl)-4-(2-oxo-1,2-dihydroquinolin-3-yl)piperidine-1-carboxamide (BMS-742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett23:3157-61 (2013) [PubMed] Article