| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50005010 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_966467 (CHEMBL2399055) |
|---|
| IC50 | 7700±n/a nM |
|---|
| Citation |  Décor, A; Grand-Maître, C; Hucke, O; O'Meara, J; Kuhn, C; Constantineau-Forget, L; Brochu, C; Malenfant, E; Bertrand-Laperle, M; Bordeleau, J; Ghiro, E; Pesant, M; Fazal, G; Gorys, V; Little, M; Boucher, C; Bordeleau, S; Turcotte, P; Guo, T; Garneau, M; Spickler, C; Gauthier, A Design, synthesis and biological evaluation of novel aminothiazoles as antiviral compounds acting against human rhinovirus. Bioorg Med Chem Lett23:3841-7 (2013) [PubMed] Article Décor, A; Grand-Maître, C; Hucke, O; O'Meara, J; Kuhn, C; Constantineau-Forget, L; Brochu, C; Malenfant, E; Bertrand-Laperle, M; Bordeleau, J; Ghiro, E; Pesant, M; Fazal, G; Gorys, V; Little, M; Boucher, C; Bordeleau, S; Turcotte, P; Guo, T; Garneau, M; Spickler, C; Gauthier, A Design, synthesis and biological evaluation of novel aminothiazoles as antiviral compounds acting against human rhinovirus. Bioorg Med Chem Lett23:3841-7 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50005010 |
|---|
| n/a |
|---|
| Name | BDBM50005010 |
|---|
| Synonyms: | CHEMBL2397313 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H19N5O2S |
|---|
| Mol. Mass. | 405.473 |
|---|
| SMILES | Cc1nc(NC(=O)Cc2nc(c(C)o2)-c2ccccc2)sc1-c1ccc(N)nc1 |
|---|
| Structure | 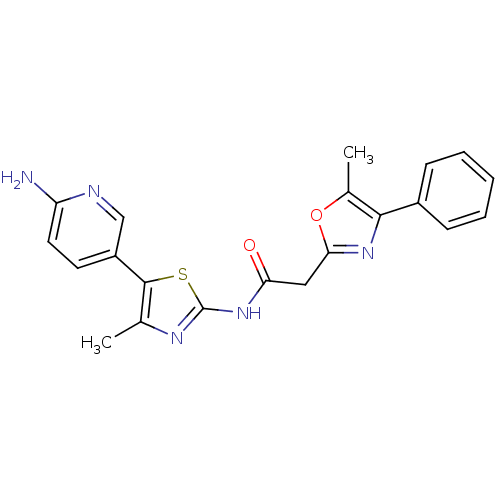
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Décor, A; Grand-Maître, C; Hucke, O; O'Meara, J; Kuhn, C; Constantineau-Forget, L; Brochu, C; Malenfant, E; Bertrand-Laperle, M; Bordeleau, J; Ghiro, E; Pesant, M; Fazal, G; Gorys, V; Little, M; Boucher, C; Bordeleau, S; Turcotte, P; Guo, T; Garneau, M; Spickler, C; Gauthier, A Design, synthesis and biological evaluation of novel aminothiazoles as antiviral compounds acting against human rhinovirus. Bioorg Med Chem Lett23:3841-7 (2013) [PubMed] Article
Décor, A; Grand-Maître, C; Hucke, O; O'Meara, J; Kuhn, C; Constantineau-Forget, L; Brochu, C; Malenfant, E; Bertrand-Laperle, M; Bordeleau, J; Ghiro, E; Pesant, M; Fazal, G; Gorys, V; Little, M; Boucher, C; Bordeleau, S; Turcotte, P; Guo, T; Garneau, M; Spickler, C; Gauthier, A Design, synthesis and biological evaluation of novel aminothiazoles as antiviral compounds acting against human rhinovirus. Bioorg Med Chem Lett23:3841-7 (2013) [PubMed] Article