| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Fatty-acid amide hydrolase 1 [30-579] |
|---|
| Ligand | BDBM50437228 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_969749 (CHEMBL2404091) |
|---|
| IC50 | 1.6±n/a nM |
|---|
| Citation |  Moreno-Sanz, G; Duranti, A; Melzig, L; Fiorelli, C; Ruda, GF; Colombano, G; Mestichelli, P; Sanchini, S; Tontini, A; Mor, M; Bandiera, T; Scarpelli, R; Tarzia, G; Piomelli, D Synthesis and structure-activity relationship studies of O-biphenyl-3-yl carbamates as peripherally restricted fatty acid amide hydrolase inhibitors. J Med Chem56:5917-30 (2014) [PubMed] Article Moreno-Sanz, G; Duranti, A; Melzig, L; Fiorelli, C; Ruda, GF; Colombano, G; Mestichelli, P; Sanchini, S; Tontini, A; Mor, M; Bandiera, T; Scarpelli, R; Tarzia, G; Piomelli, D Synthesis and structure-activity relationship studies of O-biphenyl-3-yl carbamates as peripherally restricted fatty acid amide hydrolase inhibitors. J Med Chem56:5917-30 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Fatty-acid amide hydrolase 1 [30-579] |
|---|
| Name: | Fatty-acid amide hydrolase 1 [30-579] |
|---|
| Synonyms: | Anandamide amidohydrolase 1 | FAAH1_RAT | Faah | Faah1 | Fatty Acid Amide Hydrolase | Fatty Acid Amide Hydrolic, FAAH | Fatty-acid amide hydrolase (FAAH) | Fatty-acid amide hydrolase 1 | Fatty-acid amide hydrolase 1 (FAAH) | Fatty-acid amide hydrolase 1 (aa 30-579) | Oleamide hydrolase 1 |
|---|
| Type: | Single-pass membrane protein; homodimer |
|---|
| Mol. Mass.: | 60474.00 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | P97612 (aa 30-579) |
|---|
| Residue: | 550 |
|---|
| Sequence: | RWTGRQKARGAATRARQKQRASLETMDKAVQRFRLQNPDLDSEALLTLPLLQLVQKLQSG
ELSPEAVFFTYLGKAWEVNKGTNCVTSYLTDCETQLSQAPRQGLLYGVPVSLKECFSYKG
HDSTLGLSLNEGMPSESDCVVVQVLKLQGAVPFVHTNVPQSMLSFDCSNPLFGQTMNPWK
SSKSPGGSSGGEGALIGSGGSPLGLGTDIGGSIRFPSAFCGICGLKPTGNRLSKSGLKGC
VYGQTAVQLSLGPMARDVESLALCLKALLCEHLFTLDPTVPPLPFREEVYRSSRPLRVGY
YETDNYTMPSPAMRRALIETKQRLEAAGHTLIPFLPNNIPYALEVLSAGGLFSDGGRSFL
QNFKGDFVDPCLGDLILILRLPSWFKRLLSLLLKPLFPRLAAFLNSMRPRSAEKLWKLQH
EIEMYRQSVIAQWKAMNLDVLLTPMLGPALDLNTPGRATGAISYTVLYNCLDFPAGVVPV
TTVTAEDDAQMELYKGYFGDIWDIILKKAMKNSVGLPVAVQCVALPWQEELCLRFMREVE
QLMTPQKQPS
|
|
|
|---|
| BDBM50437228 |
|---|
| n/a |
|---|
| Name | BDBM50437228 |
|---|
| Synonyms: | CHEMBL2402920 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H23NO4 |
|---|
| Mol. Mass. | 341.4009 |
|---|
| SMILES | OCc1cccc(c1)-c1cc(OC(=O)NC2CCCCC2)ccc1O |
|---|
| Structure | 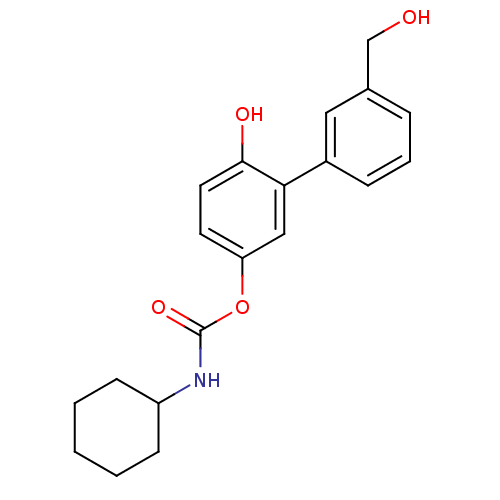
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Moreno-Sanz, G; Duranti, A; Melzig, L; Fiorelli, C; Ruda, GF; Colombano, G; Mestichelli, P; Sanchini, S; Tontini, A; Mor, M; Bandiera, T; Scarpelli, R; Tarzia, G; Piomelli, D Synthesis and structure-activity relationship studies of O-biphenyl-3-yl carbamates as peripherally restricted fatty acid amide hydrolase inhibitors. J Med Chem56:5917-30 (2014) [PubMed] Article
Moreno-Sanz, G; Duranti, A; Melzig, L; Fiorelli, C; Ruda, GF; Colombano, G; Mestichelli, P; Sanchini, S; Tontini, A; Mor, M; Bandiera, T; Scarpelli, R; Tarzia, G; Piomelli, D Synthesis and structure-activity relationship studies of O-biphenyl-3-yl carbamates as peripherally restricted fatty acid amide hydrolase inhibitors. J Med Chem56:5917-30 (2014) [PubMed] Article