| Reaction Details |
|---|
 | Report a problem with these data |
| Target | N-acylethanolamine-hydrolyzing acid amidase |
|---|
| Ligand | BDBM50439653 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_978419 (CHEMBL2421061) |
|---|
| IC50 | 5.0±n/a nM |
|---|
| Citation |  Ponzano, S; Bertozzi, F; Mengatto, L; Dionisi, M; Armirotti, A; Romeo, E; Berteotti, A; Fiorelli, C; Tarozzo, G; Reggiani, A; Duranti, A; Tarzia, G; Mor, M; Cavalli, A; Piomelli, D; Bandiera, T Synthesis and structure-activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors. J Med Chem56:6917-34 (2013) [PubMed] Article Ponzano, S; Bertozzi, F; Mengatto, L; Dionisi, M; Armirotti, A; Romeo, E; Berteotti, A; Fiorelli, C; Tarozzo, G; Reggiani, A; Duranti, A; Tarzia, G; Mor, M; Cavalli, A; Piomelli, D; Bandiera, T Synthesis and structure-activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors. J Med Chem56:6917-34 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| N-acylethanolamine-hydrolyzing acid amidase |
|---|
| Name: | N-acylethanolamine-hydrolyzing acid amidase |
|---|
| Synonyms: | ASAH-like protein | ASAHL | Acid ceramidase-like protein | N-acylethanolamine-hydrolyzing acid amidase | N-acylsphingosine amidohydrolase-like | N-acylsphingosine-amidohydrolase | NAAA | NAAA_HUMAN | PLT |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40073.12 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q02083 |
|---|
| Residue: | 359 |
|---|
| Sequence: | MRTADREARPGLPSLLLLLLAGAGLSAASPPAAPRFNVSLDSVPELRWLPVLRHYDLDLV
RAAMAQVIGDRVPKWVHVLIGKVVLELERFLPQPFTGEIRGMCDFMNLSLADCLLVNLAY
ESSVFCTSIVAQDSRGHIYHGRNLDYPFGNVLRKLTVDVQFLKNGQIAFTGTTFIGYVGL
WTGQSPHKFTVSGDERDKGWWWENAIAALFRRHIPVSWLIRATLSESENFEAAVGKLAKT
PLIADVYYIVGGTSPREGVVITRNRDGPADIWPLDPLNGAWFRVETNYDHWKPAPKEDDR
RTSAIKALNATGQANLSLEALFQILSVVPVYNNFTIYTTVMSAGSPDKYMTRIRNPSRK
|
|
|
|---|
| BDBM50439653 |
|---|
| n/a |
|---|
| Name | BDBM50439653 |
|---|
| Synonyms: | CHEMBL2419830 | US9353075, 35 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H27NO4 |
|---|
| Mol. Mass. | 297.3899 |
|---|
| SMILES | C[C@@H]1OC(=O)[C@@H]1NC(=O)OCCCCCC1CCCCC1 |r| |
|---|
| Structure | 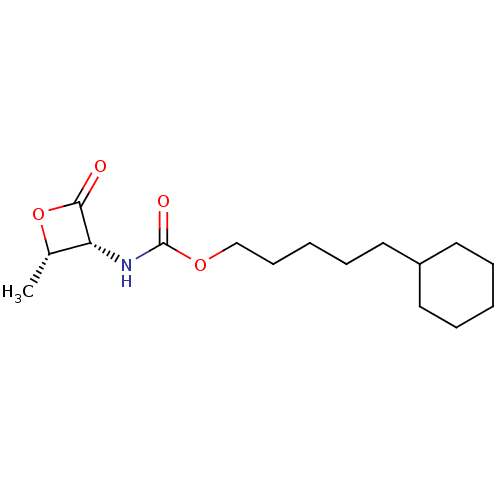
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ponzano, S; Bertozzi, F; Mengatto, L; Dionisi, M; Armirotti, A; Romeo, E; Berteotti, A; Fiorelli, C; Tarozzo, G; Reggiani, A; Duranti, A; Tarzia, G; Mor, M; Cavalli, A; Piomelli, D; Bandiera, T Synthesis and structure-activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors. J Med Chem56:6917-34 (2013) [PubMed] Article
Ponzano, S; Bertozzi, F; Mengatto, L; Dionisi, M; Armirotti, A; Romeo, E; Berteotti, A; Fiorelli, C; Tarozzo, G; Reggiani, A; Duranti, A; Tarzia, G; Mor, M; Cavalli, A; Piomelli, D; Bandiera, T Synthesis and structure-activity relationship (SAR) of 2-methyl-4-oxo-3-oxetanylcarbamic acid esters, a class of potent N-acylethanolamine acid amidase (NAAA) inhibitors. J Med Chem56:6917-34 (2013) [PubMed] Article