| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50319926 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_978835 (CHEMBL2423437) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Cano, C; Saravanan, K; Bailey, C; Bardos, J; Curtin, NJ; Frigerio, M; Golding, BT; Hardcastle, IR; Hummersone, MG; Menear, KA; Newell, DR; Richardson, CJ; Shea, K; Smith, GC; Thommes, P; Ting, A; Griffin, RJ 1-substituted (Dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chromen-4-ones endowed with dual DNA-PK/PI3-K inhibitory activity. J Med Chem56:6386-401 (2013) [PubMed] Article Cano, C; Saravanan, K; Bailey, C; Bardos, J; Curtin, NJ; Frigerio, M; Golding, BT; Hardcastle, IR; Hummersone, MG; Menear, KA; Newell, DR; Richardson, CJ; Shea, K; Smith, GC; Thommes, P; Ting, A; Griffin, RJ 1-substituted (Dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chromen-4-ones endowed with dual DNA-PK/PI3-K inhibitory activity. J Med Chem56:6386-401 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50319926 |
|---|
| n/a |
|---|
| Name | BDBM50319926 |
|---|
| Synonyms: | 2-(4-ethylpiperazin-1-yl)-N-(4-(2-morpholino-4-oxo-4H-chromen-8-yl)dibenzo[b,d]thiophen-1-yl)acetamide | CHEMBL1086377 | KU-0060648 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H34N4O4S |
|---|
| Mol. Mass. | 582.712 |
|---|
| SMILES | CCN1CCN(CC(=O)Nc2ccc(-c3cccc4c3oc(cc4=O)N3CCOCC3)c3sc4ccccc4c23)CC1 |
|---|
| Structure | 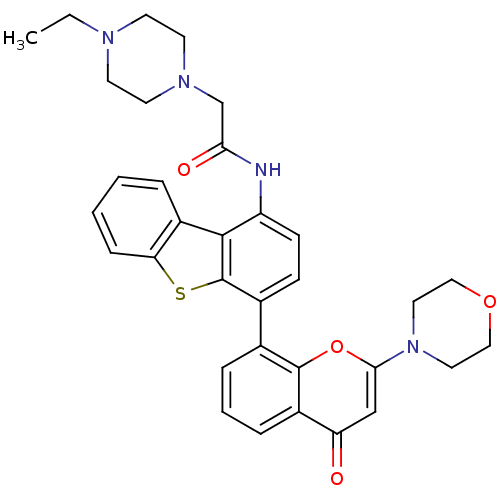
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cano, C; Saravanan, K; Bailey, C; Bardos, J; Curtin, NJ; Frigerio, M; Golding, BT; Hardcastle, IR; Hummersone, MG; Menear, KA; Newell, DR; Richardson, CJ; Shea, K; Smith, GC; Thommes, P; Ting, A; Griffin, RJ 1-substituted (Dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chromen-4-ones endowed with dual DNA-PK/PI3-K inhibitory activity. J Med Chem56:6386-401 (2013) [PubMed] Article
Cano, C; Saravanan, K; Bailey, C; Bardos, J; Curtin, NJ; Frigerio, M; Golding, BT; Hardcastle, IR; Hummersone, MG; Menear, KA; Newell, DR; Richardson, CJ; Shea, K; Smith, GC; Thommes, P; Ting, A; Griffin, RJ 1-substituted (Dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chromen-4-ones endowed with dual DNA-PK/PI3-K inhibitory activity. J Med Chem56:6386-401 (2013) [PubMed] Article