| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 5 |
|---|
| Ligand | BDBM50441722 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_988290 (CHEMBL2439744) |
|---|
| IC50 | 1.3±n/a nM |
|---|
| Citation |  Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; McEachern, E; Metz, M; Harwig, C; Li, TS; Yang, W; Bogucki, D; Zhu, Y; Langille, J; Veale, D; Ba, T; Bey, M; Baird, I; Kaller, A; Krumpak, M; Leitch, D; Satori, M; Vocadlo, K; Guay, D; Nan, S; Yee, H; Crawford, J; Chen, G; Wilson, T; Carpenter, B; Gauthier, D; Macfarland, R; Mosi, R; Bodart, V; Wong, R; Fricker, S; Schols, D Design of substituted imidazolidinylpiperidinylbenzoic acids as chemokine receptor 5 antagonists: potent inhibitors of R5 HIV-1 replication. J Med Chem56:8049-65 (2013) [PubMed] Article Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; McEachern, E; Metz, M; Harwig, C; Li, TS; Yang, W; Bogucki, D; Zhu, Y; Langille, J; Veale, D; Ba, T; Bey, M; Baird, I; Kaller, A; Krumpak, M; Leitch, D; Satori, M; Vocadlo, K; Guay, D; Nan, S; Yee, H; Crawford, J; Chen, G; Wilson, T; Carpenter, B; Gauthier, D; Macfarland, R; Mosi, R; Bodart, V; Wong, R; Fricker, S; Schols, D Design of substituted imidazolidinylpiperidinylbenzoic acids as chemokine receptor 5 antagonists: potent inhibitors of R5 HIV-1 replication. J Med Chem56:8049-65 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 5 |
|---|
| Name: | C-C chemokine receptor type 5 |
|---|
| Synonyms: | C-C CKR-5 | C-C chemokine receptor type 5 | CC-CKR-5 | CCR-5 | CCR5 | CCR5/mu opioid receptor complex | CCR5_HUMAN | CD_antigen=CD195 | CHEMR13 | CMKBR5 | HIV-1 fusion coreceptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40540.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P51681 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MDYQVSSPIYDINYYTSEPCQKINVKQIAARLLPPLYSLVFIFGFVGNMLVILILINCKR
LKSMTDIYLLNLAISDLFFLLTVPFWAHYAAAQWDFGNTMCQLLTGLYFIGFFSGIFFII
LLTIDRYLAVVHAVFALKARTVTFGVVTSVITWVVAVFASLPGIIFTRSQKEGLHYTCSS
HFPYSQYQFWKNFQTLKIVILGLVLPLLVMVICYSGILKTLLRCRNEKKRHRAVRLIFTI
MIVYFLFWAPYNIVLLLNTFQEFFGLNNCSSSNRLDQAMQVTETLGMTHCCINPIIYAFV
GEKFRNYLLVFFQKHIAKRFCKCCSIFQQEAPERASSVYTRSTGEQEISVGL
|
|
|
|---|
| BDBM50441722 |
|---|
| n/a |
|---|
| Name | BDBM50441722 |
|---|
| Synonyms: | CHEMBL2435843 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C34H41N5O4 |
|---|
| Mol. Mass. | 583.7204 |
|---|
| SMILES | CNC(=O)c1ccc(Oc2ccc(CN3CCC(CC3)N3[C@@H](CN(C4CCOCC4)C3=O)c3ccccc3)c(C)n2)cc1 |r| |
|---|
| Structure | 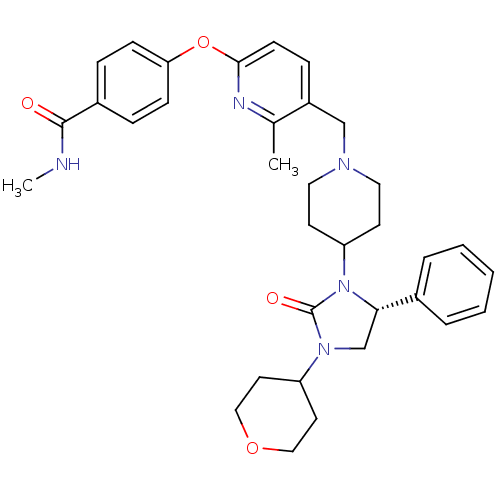
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; McEachern, E; Metz, M; Harwig, C; Li, TS; Yang, W; Bogucki, D; Zhu, Y; Langille, J; Veale, D; Ba, T; Bey, M; Baird, I; Kaller, A; Krumpak, M; Leitch, D; Satori, M; Vocadlo, K; Guay, D; Nan, S; Yee, H; Crawford, J; Chen, G; Wilson, T; Carpenter, B; Gauthier, D; Macfarland, R; Mosi, R; Bodart, V; Wong, R; Fricker, S; Schols, D Design of substituted imidazolidinylpiperidinylbenzoic acids as chemokine receptor 5 antagonists: potent inhibitors of R5 HIV-1 replication. J Med Chem56:8049-65 (2013) [PubMed] Article
Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; McEachern, E; Metz, M; Harwig, C; Li, TS; Yang, W; Bogucki, D; Zhu, Y; Langille, J; Veale, D; Ba, T; Bey, M; Baird, I; Kaller, A; Krumpak, M; Leitch, D; Satori, M; Vocadlo, K; Guay, D; Nan, S; Yee, H; Crawford, J; Chen, G; Wilson, T; Carpenter, B; Gauthier, D; Macfarland, R; Mosi, R; Bodart, V; Wong, R; Fricker, S; Schols, D Design of substituted imidazolidinylpiperidinylbenzoic acids as chemokine receptor 5 antagonists: potent inhibitors of R5 HIV-1 replication. J Med Chem56:8049-65 (2013) [PubMed] Article