| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Ligand | BDBM50441783 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_988373 (CHEMBL2437800) |
|---|
| IC50 | 1180±n/a nM |
|---|
| Citation |  Chen, YT; Tang, CL; Ma, WP; Gao, LX; Wei, Y; Zhang, W; Li, JY; Li, J; Nan, FJ Design, synthesis, and biological evaluation of novel 2-ethyl-5-phenylthiazole-4-carboxamide derivatives as protein tyrosine phosphatase 1B inhibitors with improved cellular efficacy. Eur J Med Chem69:399-412 (2013) [PubMed] Article Chen, YT; Tang, CL; Ma, WP; Gao, LX; Wei, Y; Zhang, W; Li, JY; Li, J; Nan, FJ Design, synthesis, and biological evaluation of novel 2-ethyl-5-phenylthiazole-4-carboxamide derivatives as protein tyrosine phosphatase 1B inhibitors with improved cellular efficacy. Eur J Med Chem69:399-412 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Name: | Tyrosine-protein phosphatase non-receptor type 1 |
|---|
| Synonyms: | PTN1_HUMAN | PTP1B | PTPN1 | Protein tyrosine phosphatase 1B (PTP1B) | Protein tyrosine phosphatase-1B (PTP1B) | Protein-tyrosine phosphatase 1B | Protein-tyrosine phosphatase 1B (PTP1B) | Tyrosine-protein phosphatase non-receptor type 1 | Tyrosine-protein phosphatase non-receptor type 1 (PTP1B) |
|---|
| Type: | Protein phosphatase |
|---|
| Mol. Mass.: | 49963.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human recombinant GST-fusion PTP1B (1-435). |
|---|
| Residue: | 435 |
|---|
| Sequence: | MEMEKEFEQIDKSGSWAAIYQDIRHEASDFPCRVAKLPKNKNRNRYRDVSPFDHSRIKLH
QEDNDYINASLIKMEEAQRSYILTQGPLPNTCGHFWEMVWEQKSRGVVMLNRVMEKGSLK
CAQYWPQKEEKEMIFEDTNLKLTLISEDIKSYYTVRQLELENLTTQETREILHFHYTTWP
DFGVPESPASFLNFLFKVRESGSLSPEHGPVVVHCSAGIGRSGTFCLADTCLLLMDKRKD
PSSVDIKKVLLEMRKFRMGLIQTADQLRFSYLAVIEGAKFIMGDSSVQDQWKELSHEDLE
PPPEHIPPPPRPPKRILEPHNGKCREFFPNHQWVKEETQEDKDCPIKEEKGSPLNAAPYG
IESMSQDTEVRSRVVGGSLRGAQAASPAKGEPSLPEKDEDHALSYWKPFLVNMCVATVLT
AGAYLCYRFLFNSNT
|
|
|
|---|
| BDBM50441783 |
|---|
| n/a |
|---|
| Name | BDBM50441783 |
|---|
| Synonyms: | CHEMBL2436039 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C43H35BrN4O5S |
|---|
| Mol. Mass. | 799.731 |
|---|
| SMILES | OC(=O)c1ccc(Br)cc1NC(=O)c1nc(sc1-c1ccccc1)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCc1c[nH]c2ccccc12 |r| |
|---|
| Structure | 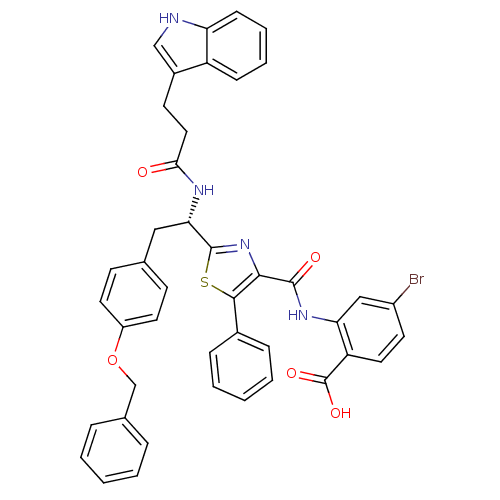
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, YT; Tang, CL; Ma, WP; Gao, LX; Wei, Y; Zhang, W; Li, JY; Li, J; Nan, FJ Design, synthesis, and biological evaluation of novel 2-ethyl-5-phenylthiazole-4-carboxamide derivatives as protein tyrosine phosphatase 1B inhibitors with improved cellular efficacy. Eur J Med Chem69:399-412 (2013) [PubMed] Article
Chen, YT; Tang, CL; Ma, WP; Gao, LX; Wei, Y; Zhang, W; Li, JY; Li, J; Nan, FJ Design, synthesis, and biological evaluation of novel 2-ethyl-5-phenylthiazole-4-carboxamide derivatives as protein tyrosine phosphatase 1B inhibitors with improved cellular efficacy. Eur J Med Chem69:399-412 (2013) [PubMed] Article