| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Leukotriene A-4 hydrolase |
|---|
| Ligand | BDBM50441912 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_987635 (CHEMBL2439368) |
|---|
| Ki | 607±n/a nM |
|---|
| Citation |  Ali, AR; El-Bendary, ER; Ghaly, MA; Shehata, IA Novel acetamidothiazole derivatives: synthesis and in vitro anticancer evaluation. Eur J Med Chem69:908-19 (2013) [PubMed] Article Ali, AR; El-Bendary, ER; Ghaly, MA; Shehata, IA Novel acetamidothiazole derivatives: synthesis and in vitro anticancer evaluation. Eur J Med Chem69:908-19 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Leukotriene A-4 hydrolase |
|---|
| Name: | Leukotriene A-4 hydrolase |
|---|
| Synonyms: | LKHA4_HUMAN | LTA-4 hydrolase | LTA4 | LTA4H | Leukotriene A(4) hydrolase | Leukotriene A-4 hydrolase (LTA4H) | Leukotriene A4 hydrolase |
|---|
| Type: | Hydrolase; metalloprotease |
|---|
| Mol. Mass.: | 69280.41 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human recombinant LTA4H. |
|---|
| Residue: | 611 |
|---|
| Sequence: | MPEIVDTCSLASPASVCRTKHLHLRCSVDFTRRTLTGTAALTVQSQEDNLRSLVLDTKDL
TIEKVVINGQEVKYALGERQSYKGSPMEISLPIALSKNQEIVIEISFETSPKSSALQWLT
PEQTSGKEHPYLFSQCQAIHCRAILPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGETP
DPEDPSRKIYKFIQKVPIPCYLIALVVGALESRQIGPRTLVWSEKEQVEKSAYEFSETES
MLKIAEDLGGPYVWGQYDLLVLPPSFPYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISH
SWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFNALGGWGELQNSVKTFGET
HPFTKLVVDLTDIDPDVAYSSVPYEKGFALLFYLEQLLGGPEIFLGFLKAYVEKFSYKSI
TTDDWKDFLYSYFKDKVDVLNQVDWNAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAK
EDDLNSFNATDLKDLSSHQLNEFLAQTLQRAPLPLGHIKRMQEVYNFNAINNSEIRFRWL
RLCIQSKWEDAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAVRTYQEHKASMHPVT
AMLVGKDLKVD
|
|
|
|---|
| BDBM50441912 |
|---|
| n/a |
|---|
| Name | BDBM50441912 |
|---|
| Synonyms: | CHEMBL2437295 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H19ClN4O2S |
|---|
| Mol. Mass. | 378.876 |
|---|
| SMILES | CC(=O)N1CCN(CC1)C(=O)Cn1c(csc1=N)-c1ccc(Cl)cc1 |
|---|
| Structure | 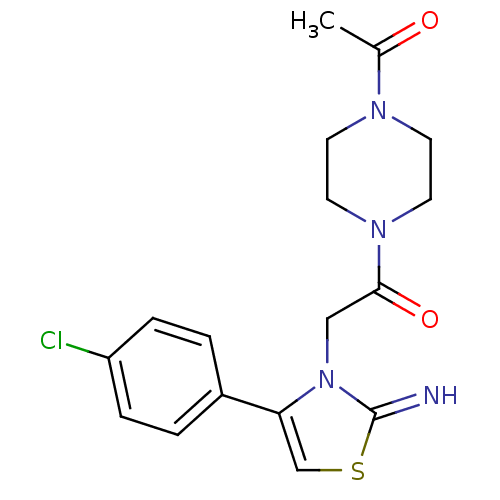
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ali, AR; El-Bendary, ER; Ghaly, MA; Shehata, IA Novel acetamidothiazole derivatives: synthesis and in vitro anticancer evaluation. Eur J Med Chem69:908-19 (2013) [PubMed] Article
Ali, AR; El-Bendary, ER; Ghaly, MA; Shehata, IA Novel acetamidothiazole derivatives: synthesis and in vitro anticancer evaluation. Eur J Med Chem69:908-19 (2013) [PubMed] Article