| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Proteasome subunit beta type-1 |
|---|
| Ligand | BDBM50069985 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1282983 (CHEMBL3100302) |
|---|
| IC50 | 120±n/a nM |
|---|
| Citation |  Hasegawa, M; Yasuda, Y; Tanaka, M; Nakata, K; Umeda, E; Wang, Y; Watanabe, C; Uetake, S; Kunoh, T; Shionyu, M; Sasaki, R; Shiina, I; Mizukami, T A novel tamoxifen derivative, ridaifen-F, is a nonpeptidic small-molecule proteasome inhibitor. Eur J Med Chem71:290-305 (2014) [PubMed] Article Hasegawa, M; Yasuda, Y; Tanaka, M; Nakata, K; Umeda, E; Wang, Y; Watanabe, C; Uetake, S; Kunoh, T; Shionyu, M; Sasaki, R; Shiina, I; Mizukami, T A novel tamoxifen derivative, ridaifen-F, is a nonpeptidic small-molecule proteasome inhibitor. Eur J Med Chem71:290-305 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Proteasome subunit beta type-1 |
|---|
| Name: | Proteasome subunit beta type-1 |
|---|
| Synonyms: | PSB1_HUMAN | PSC5 | PSMB1 | Proteasome component C5 | Proteasome subunit beta type-1/beta type-5 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 26493.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1366691 |
|---|
| Residue: | 241 |
|---|
| Sequence: | MLSSTAMYSAPGRDLGMEPHRAAGPLQLRFSPYVFNGGTILAIAGEDFAIVASDTRLSEG
FSIHTRDSPKCYKLTDKTVIGCSGFHGDCLTLTKIIEARLKMYKHSNNKAMTTGAIAAML
STILYSRRFFPYYVYNIIGGLDEEGKGAVYSFDPVGSYQRDSFKAGGSASAMLQPLLDNQ
VGFKNMQNVEHVPLSLDRAMRLVKDVFISAAERDVYTGDALRICIVTKEGIREETVSLRK
D
|
|
|
|---|
| BDBM50069985 |
|---|
| n/a |
|---|
| Name | BDBM50069985 |
|---|
| Synonyms: | (S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic acid [(S)-1-((S)-1-formyl-3-methyl-butylcarbamoyl)-3-methyl-butyl]-amide | CHEMBL64925 | Cbz-L-leu-L-leu-L-leu-CHO | MG-13 | MG-132 | Z-L-leu-L-leu-L-leu-H | Z-Leu-Leu-Leu-H | Z-Leu-Leu-Leu-al | benzyl (S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate | benzyl(S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate | benzyloxycarbonyl-Leu-Leu-leucinal | {(S)-1-[(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester | {1-[(S)-(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester | {1-[1-(1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H41N3O5 |
|---|
| Mol. Mass. | 475.6208 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| |
|---|
| Structure | 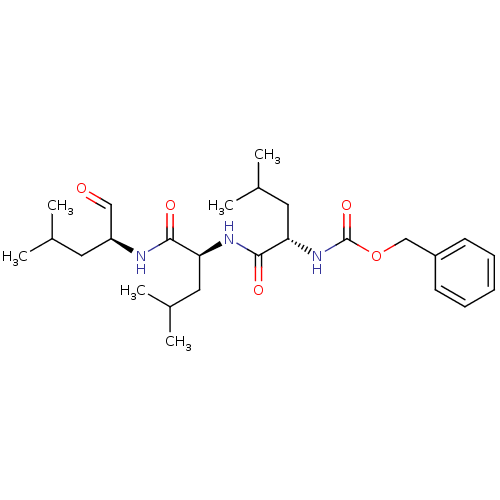
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hasegawa, M; Yasuda, Y; Tanaka, M; Nakata, K; Umeda, E; Wang, Y; Watanabe, C; Uetake, S; Kunoh, T; Shionyu, M; Sasaki, R; Shiina, I; Mizukami, T A novel tamoxifen derivative, ridaifen-F, is a nonpeptidic small-molecule proteasome inhibitor. Eur J Med Chem71:290-305 (2014) [PubMed] Article
Hasegawa, M; Yasuda, Y; Tanaka, M; Nakata, K; Umeda, E; Wang, Y; Watanabe, C; Uetake, S; Kunoh, T; Shionyu, M; Sasaki, R; Shiina, I; Mizukami, T A novel tamoxifen derivative, ridaifen-F, is a nonpeptidic small-molecule proteasome inhibitor. Eur J Med Chem71:290-305 (2014) [PubMed] Article