| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urotensin-2 receptor |
|---|
| Ligand | BDBM50378580 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1285483 (CHEMBL3108415) |
|---|
| IC50 | 13±n/a nM |
|---|
| Citation |  Chatenet, D; Folch, B; Feytens, D; Létourneau, M; Tourwé, D; Doucet, N; Fournier, A Development and pharmacological characterization of conformationally constrained urotensin II-related peptide agonists. J Med Chem56:9612-22 (2014) [PubMed] Article Chatenet, D; Folch, B; Feytens, D; Létourneau, M; Tourwé, D; Doucet, N; Fournier, A Development and pharmacological characterization of conformationally constrained urotensin II-related peptide agonists. J Med Chem56:9612-22 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urotensin-2 receptor |
|---|
| Name: | Urotensin-2 receptor |
|---|
| Synonyms: | G-protein coupled receptor 14 | GPR14 | UR-II-R | UR2R_HUMAN | UTS2R | Urotensin II receptor | Urotensin-II |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 42159.71 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Urotensin-II UTS2R HUMAN::Q9UKP6 |
|---|
| Residue: | 389 |
|---|
| Sequence: | MALTPESPSSFPGLAATGSSVPEPPGGPNATLNSSWASPTEPSSLEDLVATGTIGTLLSA
MGVVGVVGNAYTLVVTCRSLRAVASMYVYVVNLALADLLYLLSIPFIVATYVTKEWHFGD
VGCRVLFGLDFLTMHASIFTLTVMSSERYAAVLRPLDTVQRPKGYRKLLALGTWLLALLL
TLPVMLAMRLVRRGPKSLCLPAWGPRAHRAYLTLLFATSIAGPGLLIGLLYARLARAYRR
SQRASFKRARRPGARALRLVLGIVLLFWACFLPFWLWQLLAQYHQAPLAPRTARIVNYLT
TCLTYGNSCANPFLYTLLTRNYRDHLRGRVRGPGSGGGRGPVPSLQPRARFQRCSGRSLS
SCSPQPTDSLVLAPAAPARPAPEGPRAPA
|
|
|
|---|
| BDBM50378580 |
|---|
| n/a |
|---|
| Name | BDBM50378580 |
|---|
| Synonyms: | CHEMBL437430 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C64H85N13O18S2 |
|---|
| Mol. Mass. | 1388.566 |
|---|
| SMILES | CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |
|---|
| Structure | 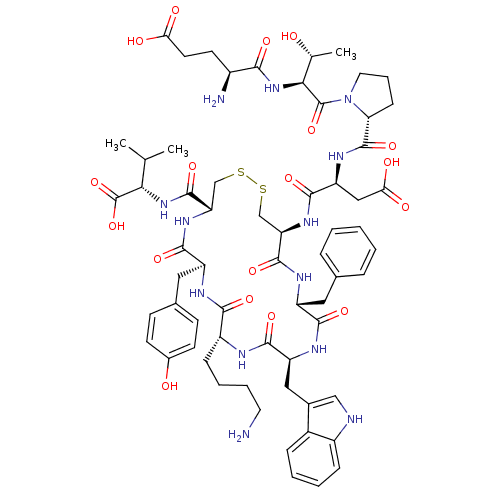
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chatenet, D; Folch, B; Feytens, D; Létourneau, M; Tourwé, D; Doucet, N; Fournier, A Development and pharmacological characterization of conformationally constrained urotensin II-related peptide agonists. J Med Chem56:9612-22 (2014) [PubMed] Article
Chatenet, D; Folch, B; Feytens, D; Létourneau, M; Tourwé, D; Doucet, N; Fournier, A Development and pharmacological characterization of conformationally constrained urotensin II-related peptide agonists. J Med Chem56:9612-22 (2014) [PubMed] Article