

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Adenosine receptor A1 | ||
| Ligand | BDBM50364063 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1352075 (CHEMBL3267340) | ||
| Ki | 77±n/a nM | ||
| Citation |  de Lera Ruiz, M; Lim, YH; Zheng, J Adenosine A2A receptor as a drug discovery target. J Med Chem57:3623-50 (2014) [PubMed] Article de Lera Ruiz, M; Lim, YH; Zheng, J Adenosine A2A receptor as a drug discovery target. J Med Chem57:3623-50 (2014) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Adenosine receptor A1 | |||
| Name: | Adenosine receptor A1 | ||
| Synonyms: | A1 adenosine receptor (hA1) | A1AR | AA1R_HUMAN | ADENOSINE A1 | ADORA1 | Adenosine A1 receptor (A1AR) | Adenosine A1-receptor | Adenosine receptor A1 (A1) | Adenosine receptor A1 (hA1) | Adenosine transporter (AdT) | ||
| Type: | G Protein-Coupled Receptor (GPCR) | ||
| Mol. Mass.: | 36520.92 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P30542 | ||
| Residue: | 326 | ||
| Sequence: |
| ||
| BDBM50364063 | |||
| n/a | |||
| Name | BDBM50364063 | ||
| Synonyms: | CHEMBL1950649 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C23H30N6O6 | ||
| Mol. Mass. | 486.5209 | ||
| SMILES | CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(nc12)C#CC[C@H]1CC[C@@H](CC1)C(=O)OC |r,wU:7.12,5.4,28.34,wD:8.8,10.11,25.27,(-2.37,-48.53,;-1.04,-49.31,;.3,-48.55,;1.62,-49.33,;1.61,-50.87,;2.93,-48.59,;2.94,-47.1,;4.43,-46.64,;5.27,-47.83,;6.72,-47.84,;4.37,-49.06,;4.85,-50.53,;4.89,-45.21,;3.96,-43.88,;4.91,-42.6,;6.23,-43.05,;7.53,-42.29,;7.52,-40.78,;8.97,-43.12,;8.97,-44.75,;7.59,-45.54,;6.24,-44.75,;10.31,-45.52,;11.64,-46.29,;12.97,-47.06,;14.3,-46.29,;15.63,-47.07,;16.96,-46.31,;16.97,-44.77,;15.64,-43.99,;14.3,-44.76,;18.31,-44,;18.32,-42.46,;19.64,-44.78,;20.98,-44.02,)| | ||
| Structure | 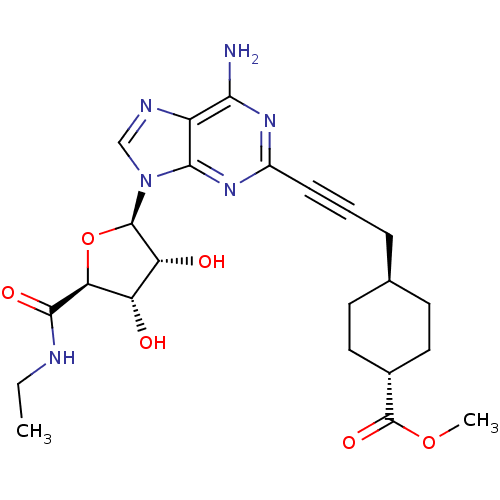
| ||