| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine/threonine-protein kinase receptor R3 |
|---|
| Ligand | BDBM50015639 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1349302 (CHEMBL3268802) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Jin, CH; Krishnaiah, M; Sreenu, D; Subrahmanyam, VB; Rao, KS; Lee, HJ; Park, SJ; Park, HJ; Lee, K; Sheen, YY; Kim, DK Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-ß type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem57:4213-38 (2014) [PubMed] Article Jin, CH; Krishnaiah, M; Sreenu, D; Subrahmanyam, VB; Rao, KS; Lee, HJ; Park, SJ; Park, HJ; Lee, K; Sheen, YY; Kim, DK Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-ß type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem57:4213-38 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Serine/threonine-protein kinase receptor R3 |
|---|
| Name: | Serine/threonine-protein kinase receptor R3 |
|---|
| Synonyms: | ACVL1_HUMAN | ACVRL1 | ACVRLK1 | ALK1 | Activin receptor-like kinase 1 (ALK-1) | Serine/threonine-protein kinase receptor R3 (ALK1) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 56134.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P37023 |
|---|
| Residue: | 503 |
|---|
| Sequence: | MTLGSPRKGLLMLLMALVTQGDPVKPSRGPLVTCTCESPHCKGPTCRGAWCTVVLVREEG
RHPQEHRGCGNLHRELCRGRPTEFVNHYCCDSHLCNHNVSLVLEATQPPSEQPGTDGQLA
LILGPVLALLALVALGVLGLWHVRRRQEKQRGLHSELGESSLILKASEQGDSMLGDLLDS
DCTTGSGSGLPFLVQRTVARQVALVECVGKGRYGEVWRGLWHGESVAVKIFSSRDEQSWF
RETEIYNTVLLRHDNILGFIASDMTSRNSSTQLWLITHYHEHGSLYDFLQRQTLEPHLAL
RLAVSAACGLAHLHVEIFGTQGKPAIAHRDFKSRNVLVKSNLQCCIADLGLAVMHSQGSD
YLDIGNNPRVGTKRYMAPEVLDEQIRTDCFESYKWTDIWAFGLVLWEIARRTIVNGIVED
YRPPFYDVVPNDPSFEDMKKVVCVDQQTPTIPNRLAADPVLSGLAQMMRECWYPNPSARL
TALRIKKTLQKISNSPEKPKVIQ
|
|
|
|---|
| BDBM50015639 |
|---|
| n/a |
|---|
| Name | BDBM50015639 |
|---|
| Synonyms: | CHEMBL3260567 | USRE47141, Example 2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H18FN7 |
|---|
| Mol. Mass. | 399.4236 |
|---|
| SMILES | Cc1cccc(n1)-c1[nH]c(CNc2ccccc2F)nc1-c1ccc2ncnn2c1 |
|---|
| Structure | 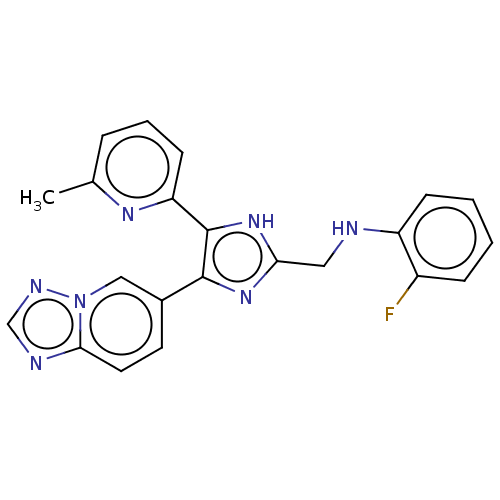
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jin, CH; Krishnaiah, M; Sreenu, D; Subrahmanyam, VB; Rao, KS; Lee, HJ; Park, SJ; Park, HJ; Lee, K; Sheen, YY; Kim, DK Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-ß type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem57:4213-38 (2014) [PubMed] Article
Jin, CH; Krishnaiah, M; Sreenu, D; Subrahmanyam, VB; Rao, KS; Lee, HJ; Park, SJ; Park, HJ; Lee, K; Sheen, YY; Kim, DK Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-ß type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem57:4213-38 (2014) [PubMed] Article