| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neurotensin receptor type 2 |
|---|
| Ligand | BDBM50240845 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1364516 (CHEMBL3295313) |
|---|
| IC50 | 5.4±n/a nM |
|---|
| Citation |  Thomas, JB; Giddings, AM; Wiethe, RW; Olepu, S; Warner, KR; Sarret, P; Gendron, L; Longpre, JM; Zhang, Y; Runyon, SP; Gilmour, BP Identification of 1-({[1-(4-fluorophenyl)-5-(2-methoxyphenyl)-1H-pyrazol-3-yl]carbonyl}amino)cyclohexane carboxylic acid as a selective nonpeptide neurotensin receptor type 2 compound. J Med Chem57:5318-32 (2014) [PubMed] Article Thomas, JB; Giddings, AM; Wiethe, RW; Olepu, S; Warner, KR; Sarret, P; Gendron, L; Longpre, JM; Zhang, Y; Runyon, SP; Gilmour, BP Identification of 1-({[1-(4-fluorophenyl)-5-(2-methoxyphenyl)-1H-pyrazol-3-yl]carbonyl}amino)cyclohexane carboxylic acid as a selective nonpeptide neurotensin receptor type 2 compound. J Med Chem57:5318-32 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neurotensin receptor type 2 |
|---|
| Name: | Neurotensin receptor type 2 |
|---|
| Synonyms: | High-affinity levocabastine-sensitive neurotensin receptor | NT-R-2 | NTR2_RAT | Neurotensin receptor 2 | Neurotensin receptor type 2 | Ntr2 | Ntsr2 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 46280.50 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_1466615 |
|---|
| Residue: | 416 |
|---|
| Sequence: | METSSPWPPRPSPSAGLSLEARLGVDTRLWAKVLFTALYSLIFAFGTAGNALSVHVVLKA
RAGRPGRLRYHVLSLALSALLLLLVSMPMELYNFVWSHYPWVFGDLGCRGYYFVRELCAY
ATVLSVASLSAERCLAVCQPLRARRLLTPRRTRRLLSLVWVASLGLALPMAVIMGQKHEV
ESADGEPEPASRVCTVLVSRATLQVFIQVNVLVSFALPLALTAFLNGITVNHLMALYSQV
PSASAQVSSIPSRLELLSEEGLLGFITWRKTLSLGVQASLVRHKDASQIRSLQHSAQVLR
AIVAVYVICWLPYHARRLMYCYIPDDGWTNELYDFYHYFYMVTNTLFYVSSAVTPILYNA
VSSSFRKLFLESLGSLCGEQHSLVPLPQEAPESTTSTYSFRLWGSPRNPSLGEIQV
|
|
|
|---|
| BDBM50240845 |
|---|
| n/a |
|---|
| Name | BDBM50240845 |
|---|
| Synonyms: | (S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amino-5-guanidino-pentanoylamino)-5-guanidino-pentanoyl]-pyrrolidine-2-carbonyl}-amino)-3-(4-hydroxy-phenyl)-propionylamino]-3-methyl-pentanoylamino}-4-methyl-pentanoic acid | 2-{(S)-2-[2-({(S)-1-[(S)-2-((S)-2-Amino-5-guanidino-pentanoylamino)-5-guanidino-pentanoyl]-pyrrolidine-2-carbonyl}-amino)-3-(4-hydroxy-phenyl)-propionylamino]-3-methyl-pentanoylamino}-4-(S)-methyl-pentanoic acid | CHEMBL342252 | Neurotensin(8-13) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C38H64N12O8 |
|---|
| Mol. Mass. | 816.9904 |
|---|
| SMILES | [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| |
|---|
| Structure | 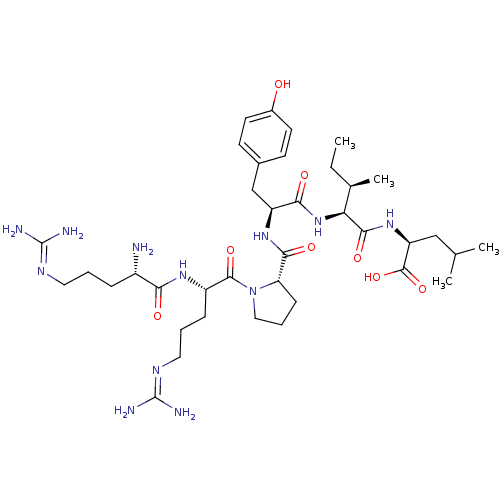
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Thomas, JB; Giddings, AM; Wiethe, RW; Olepu, S; Warner, KR; Sarret, P; Gendron, L; Longpre, JM; Zhang, Y; Runyon, SP; Gilmour, BP Identification of 1-({[1-(4-fluorophenyl)-5-(2-methoxyphenyl)-1H-pyrazol-3-yl]carbonyl}amino)cyclohexane carboxylic acid as a selective nonpeptide neurotensin receptor type 2 compound. J Med Chem57:5318-32 (2014) [PubMed] Article
Thomas, JB; Giddings, AM; Wiethe, RW; Olepu, S; Warner, KR; Sarret, P; Gendron, L; Longpre, JM; Zhang, Y; Runyon, SP; Gilmour, BP Identification of 1-({[1-(4-fluorophenyl)-5-(2-methoxyphenyl)-1H-pyrazol-3-yl]carbonyl}amino)cyclohexane carboxylic acid as a selective nonpeptide neurotensin receptor type 2 compound. J Med Chem57:5318-32 (2014) [PubMed] Article