| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50021261 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1366300 (CHEMBL3297633) |
|---|
| IC50 | 17000±n/a nM |
|---|
| Citation |  Fader, LD; Carson, R; Morin, S; Bilodeau, F; Chabot, C; Halmos, T; Bailey, MD; Kawai, SH; Coulombe, R; Laplante, S; Mekhssian, K; Jakalian, A; Garneau, M; Duan, J; Mason, SW; Simoneau, B; Fenwick, C; Tsantrizos, Y; Yoakim, C Minimizing the Contribution of Enterohepatic Recirculation to Clearance in Rat for the NCINI Class of Inhibitors of HIV. ACS Med Chem Lett5:711-6 (2014) [PubMed] Article Fader, LD; Carson, R; Morin, S; Bilodeau, F; Chabot, C; Halmos, T; Bailey, MD; Kawai, SH; Coulombe, R; Laplante, S; Mekhssian, K; Jakalian, A; Garneau, M; Duan, J; Mason, SW; Simoneau, B; Fenwick, C; Tsantrizos, Y; Yoakim, C Minimizing the Contribution of Enterohepatic Recirculation to Clearance in Rat for the NCINI Class of Inhibitors of HIV. ACS Med Chem Lett5:711-6 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50021261 |
|---|
| n/a |
|---|
| Name | BDBM50021261 |
|---|
| Synonyms: | CHEMBL3287925 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H31ClN2O4 |
|---|
| Mol. Mass. | 519.031 |
|---|
| SMILES | Cc1noc(C)c1-c1ccc(cc1)-c1nc(C)c([C@H](OC(C)(C)C)C(O)=O)c(-c2ccc(Cl)cc2)c1C |r| |
|---|
| Structure | 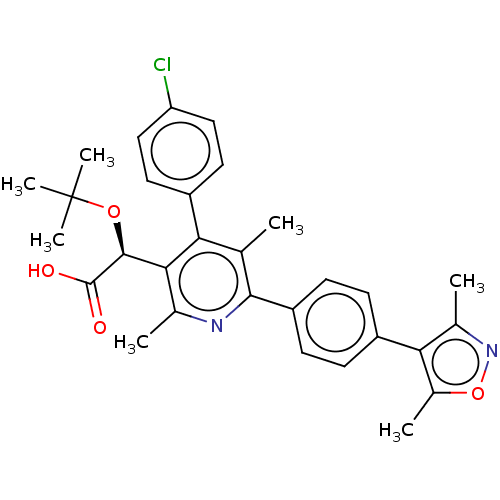
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fader, LD; Carson, R; Morin, S; Bilodeau, F; Chabot, C; Halmos, T; Bailey, MD; Kawai, SH; Coulombe, R; Laplante, S; Mekhssian, K; Jakalian, A; Garneau, M; Duan, J; Mason, SW; Simoneau, B; Fenwick, C; Tsantrizos, Y; Yoakim, C Minimizing the Contribution of Enterohepatic Recirculation to Clearance in Rat for the NCINI Class of Inhibitors of HIV. ACS Med Chem Lett5:711-6 (2014) [PubMed] Article
Fader, LD; Carson, R; Morin, S; Bilodeau, F; Chabot, C; Halmos, T; Bailey, MD; Kawai, SH; Coulombe, R; Laplante, S; Mekhssian, K; Jakalian, A; Garneau, M; Duan, J; Mason, SW; Simoneau, B; Fenwick, C; Tsantrizos, Y; Yoakim, C Minimizing the Contribution of Enterohepatic Recirculation to Clearance in Rat for the NCINI Class of Inhibitors of HIV. ACS Med Chem Lett5:711-6 (2014) [PubMed] Article