| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Muscarinic acetylcholine receptor M2 |
|---|
| Ligand | BDBM50023229 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1457831 (CHEMBL3370309) |
|---|
| EC50 | >30000±n/a nM |
|---|
| Citation |  Gentry, PR; Kokubo, M; Bridges, TM; Noetzel, MJ; Cho, HP; Lamsal, A; Smith, E; Chase, P; Hodder, PS; Niswender, CM; Daniels, JS; Conn, PJ; Lindsley, CW; Wood, MR Development of a highly potent, novel M5 positive allosteric modulator (PAM) demonstrating CNS exposure: 1-((1H-indazol-5-yl)sulfoneyl)-N-ethyl-N-(2-(trifluoromethyl)benzyl)piperidine-4-carboxamide (ML380). J Med Chem57:7804-10 (2014) [PubMed] Article Gentry, PR; Kokubo, M; Bridges, TM; Noetzel, MJ; Cho, HP; Lamsal, A; Smith, E; Chase, P; Hodder, PS; Niswender, CM; Daniels, JS; Conn, PJ; Lindsley, CW; Wood, MR Development of a highly potent, novel M5 positive allosteric modulator (PAM) demonstrating CNS exposure: 1-((1H-indazol-5-yl)sulfoneyl)-N-ethyl-N-(2-(trifluoromethyl)benzyl)piperidine-4-carboxamide (ML380). J Med Chem57:7804-10 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Muscarinic acetylcholine receptor M2 |
|---|
| Name: | Muscarinic acetylcholine receptor M2 |
|---|
| Synonyms: | ACM2_RAT | Cholinergic, muscarinic M2 | Chrm-2 | Chrm2 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 51555.53 |
|---|
| Organism: | RAT |
|---|
| Description: | P10980 |
|---|
| Residue: | 466 |
|---|
| Sequence: | MNNSTNSSNNGLAITSPYKTFEVVFIVLVAGSLSLVTIIGNILVMVSIKVNRHLQTVNNY
FLFSLACADLIIGVFSMNLYTLYTVIGYWPLGPVVCDLWLALDYVVSNASVMNLLIISFD
RYFCVTKPLTYPVKRTTKMAGMMIAAAWVLSFILWAPAILFWQFIVGVRTVEDGECYIQF
FSNAAVTFGTAIAAFYLPVIIMTVLYWHISRASKSRIKKEKKEPVANQDPVSPSLVQGRI
VKPNNNNMPGGDGGLEHNKIQNGKAPRDGVTENCVQGEEKESSNDSTSVSAVASNMRDDE
ITQDENTVSTSLGHSRDDNSKQTCIKIVTKAQKGDVCTPTSTTVELVGSSGQNGDEKQNI
VARKIVKMTKQPAKKKPPPSREKKVTRTILAILLAFIITWAPYNVMVLINTFCAPCIPNT
VWTIGYWLCYINSTINPACYALCNATFKKTFKHLLMCHYKNIGATR
|
|
|
|---|
| BDBM50023229 |
|---|
| n/a |
|---|
| Name | BDBM50023229 |
|---|
| Synonyms: | CHEMBL3329755 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H25F3N4O3S |
|---|
| Mol. Mass. | 494.53 |
|---|
| SMILES | CCN(Cc1ccccc1C(F)(F)F)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc2[nH]ncc2c1 |
|---|
| Structure | 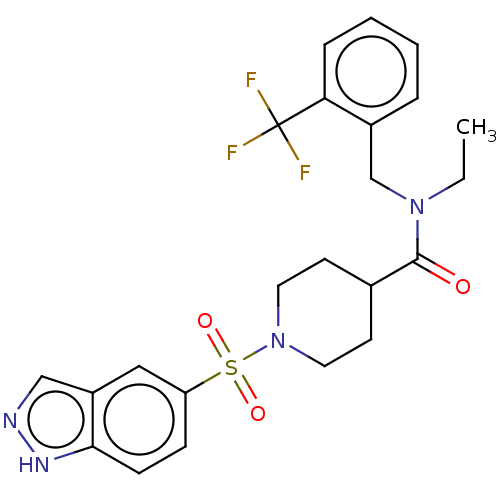
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gentry, PR; Kokubo, M; Bridges, TM; Noetzel, MJ; Cho, HP; Lamsal, A; Smith, E; Chase, P; Hodder, PS; Niswender, CM; Daniels, JS; Conn, PJ; Lindsley, CW; Wood, MR Development of a highly potent, novel M5 positive allosteric modulator (PAM) demonstrating CNS exposure: 1-((1H-indazol-5-yl)sulfoneyl)-N-ethyl-N-(2-(trifluoromethyl)benzyl)piperidine-4-carboxamide (ML380). J Med Chem57:7804-10 (2014) [PubMed] Article
Gentry, PR; Kokubo, M; Bridges, TM; Noetzel, MJ; Cho, HP; Lamsal, A; Smith, E; Chase, P; Hodder, PS; Niswender, CM; Daniels, JS; Conn, PJ; Lindsley, CW; Wood, MR Development of a highly potent, novel M5 positive allosteric modulator (PAM) demonstrating CNS exposure: 1-((1H-indazol-5-yl)sulfoneyl)-N-ethyl-N-(2-(trifluoromethyl)benzyl)piperidine-4-carboxamide (ML380). J Med Chem57:7804-10 (2014) [PubMed] Article