| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A3 |
|---|
| Ligand | BDBM50041691 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1446307 (CHEMBL3373701) |
|---|
| IC50 | 3300±n/a nM |
|---|
| Citation |  Buzard, DJ; Kim, SH; Lopez, L; Kawasaki, A; Zhu, X; Moody, J; Thoresen, L; Calderon, I; Ullman, B; Han, S; Lehmann, J; Gharbaoui, T; Sengupta, D; Calvano, L; Montalban, AG; Ma, YA; Sage, C; Gao, Y; Semple, G; Edwards, J; Barden, J; Morgan, M; Chen, W; Usmani, K; Chen, C; Sadeque, A; Christopher, RJ; Thatte, J; Fu, L; Solomon, M; Mills, D; Whelan, K; Al-Shamma, H; Gatlin, J; Le, M; Gaidarov, I; Anthony, T; Unett, DJ; Blackburn, A; Rueter, J; Stirn, S; Behan, DP; Jones, RM Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. ACS Med Chem Lett5:1313-7 (2014) [PubMed] Article Buzard, DJ; Kim, SH; Lopez, L; Kawasaki, A; Zhu, X; Moody, J; Thoresen, L; Calderon, I; Ullman, B; Han, S; Lehmann, J; Gharbaoui, T; Sengupta, D; Calvano, L; Montalban, AG; Ma, YA; Sage, C; Gao, Y; Semple, G; Edwards, J; Barden, J; Morgan, M; Chen, W; Usmani, K; Chen, C; Sadeque, A; Christopher, RJ; Thatte, J; Fu, L; Solomon, M; Mills, D; Whelan, K; Al-Shamma, H; Gatlin, J; Le, M; Gaidarov, I; Anthony, T; Unett, DJ; Blackburn, A; Rueter, J; Stirn, S; Behan, DP; Jones, RM Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. ACS Med Chem Lett5:1313-7 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A3 |
|---|
| Name: | Adenosine receptor A3 |
|---|
| Synonyms: | A3 adenosine receptor (hA3) | AA3R_HUMAN | ADORA3 | Adenosine A3 receptor (A3AR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 36197.32 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P0DMS8 |
|---|
| Residue: | 318 |
|---|
| Sequence: | MPNNSTALSLANVTYITMEIFIGLCAIVGNVLVICVVKLNPSLQTTTFYFIVSLALADIA
VGVLVMPLAIVVSLGITIHFYSCLFMTCLLLIFTHASIMSLLAIAVDRYLRVKLTVRYKR
VTTHRRIWLALGLCWLVSFLVGLTPMFGWNMKLTSEYHRNVTFLSCQFVSVMRMDYMVYF
SFLTWIFIPLVVMCAIYLDIFYIIRNKLSLNLSNSKETGAFYGREFKTAKSLFLVLFLFA
LSWLPLSIINCIIYFNGEVPQLVLYMGILLSHANSMMNPIVYAYKIKKFKETYLLILKAC
VVCHPSDSLDTSIEKNSE
|
|
|
|---|
| BDBM50041691 |
|---|
| n/a |
|---|
| Name | BDBM50041691 |
|---|
| Synonyms: | CHEMBL3358920 | US11149292, Compound (R)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)- |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H26F3NO3 |
|---|
| Mol. Mass. | 457.4847 |
|---|
| SMILES | OC(=O)C[C@H]1CCc2c1[nH]c1ccc(OCc3ccc(C4CCCC4)c(c3)C(F)(F)F)cc21 |r| |
|---|
| Structure | 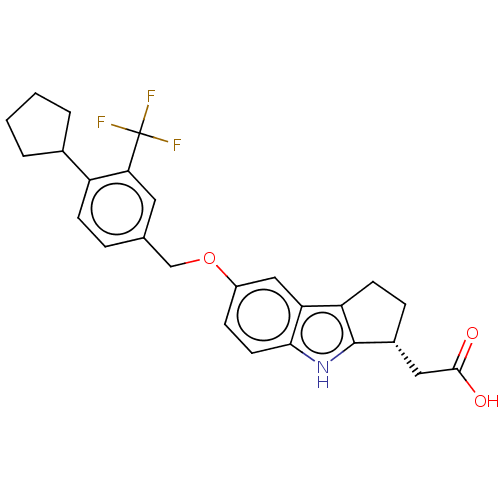
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Buzard, DJ; Kim, SH; Lopez, L; Kawasaki, A; Zhu, X; Moody, J; Thoresen, L; Calderon, I; Ullman, B; Han, S; Lehmann, J; Gharbaoui, T; Sengupta, D; Calvano, L; Montalban, AG; Ma, YA; Sage, C; Gao, Y; Semple, G; Edwards, J; Barden, J; Morgan, M; Chen, W; Usmani, K; Chen, C; Sadeque, A; Christopher, RJ; Thatte, J; Fu, L; Solomon, M; Mills, D; Whelan, K; Al-Shamma, H; Gatlin, J; Le, M; Gaidarov, I; Anthony, T; Unett, DJ; Blackburn, A; Rueter, J; Stirn, S; Behan, DP; Jones, RM Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. ACS Med Chem Lett5:1313-7 (2014) [PubMed] Article
Buzard, DJ; Kim, SH; Lopez, L; Kawasaki, A; Zhu, X; Moody, J; Thoresen, L; Calderon, I; Ullman, B; Han, S; Lehmann, J; Gharbaoui, T; Sengupta, D; Calvano, L; Montalban, AG; Ma, YA; Sage, C; Gao, Y; Semple, G; Edwards, J; Barden, J; Morgan, M; Chen, W; Usmani, K; Chen, C; Sadeque, A; Christopher, RJ; Thatte, J; Fu, L; Solomon, M; Mills, D; Whelan, K; Al-Shamma, H; Gatlin, J; Le, M; Gaidarov, I; Anthony, T; Unett, DJ; Blackburn, A; Rueter, J; Stirn, S; Behan, DP; Jones, RM Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. ACS Med Chem Lett5:1313-7 (2014) [PubMed] Article