| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Thioredoxin reductase 3 |
|---|
| Ligand | BDBM50384998 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1473858 (CHEMBL3418935) |
|---|
| IC50 | >40000±n/a nM |
|---|
| Citation |  Zhang, B; Duan, D; Ge, C; Yao, J; Liu, Y; Li, X; Fang, J Synthesis of xanthohumol analogues and discovery of potent thioredoxin reductase inhibitor as potential anticancer agent. J Med Chem58:1795-805 (2015) [PubMed] Article Zhang, B; Duan, D; Ge, C; Yao, J; Liu, Y; Li, X; Fang, J Synthesis of xanthohumol analogues and discovery of potent thioredoxin reductase inhibitor as potential anticancer agent. J Med Chem58:1795-805 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Thioredoxin reductase 3 |
|---|
| Name: | Thioredoxin reductase 3 |
|---|
| Synonyms: | TGR | TGR | TRXR3 | TRXR3_HUMAN | TXNRD3 | Thioredoxin and glutathione reductase | Thioredoxin reductase 3 | Thioredoxin reductase TR2 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 70543.23 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_104858 |
|---|
| Residue: | 642 |
|---|
| Sequence: | MERSPPQSPGPGKAGDAPNRRSGHVRGARVLSPPGRRARLSSPGPSRSSEAREELRRHLV
GLIERSRVVIFSKSYCPHSTRVKELFSSLGVECNVLELDQVDDGARVQEVLSEITNQKTV
PNIFVNKVHVGGCDQTFQAYQSGLLQKLLQEDLAYDYDLIIIGGGSGGLSCAKEAAILGK
KVMVLDFVVPSPQGTSWGLGGTCVNVGCIPKKLMHQAALLGQALCDSRKFGWEYNQQVRH
NWETMTKAIQNHISSLNWGYRLSLREKAVAYVNSYGEFVEHHKIKATNKKGQETYYTAAQ
FVIATGERPRYLGIQGDKEYCITSDDLFSLPYCPGKTLVVGASYVALECAGFLAGFGLDV
TVMVRSILLRGFDQEMAEKVGSYMEQHGVKFLRKFIPVMVQQLEKGSPGKLKVLAKSTEG
TETIEGVYNTVLLAIGRDSCTRKIGLEKIGVKINEKSGKIPVNDVEQTNVPYVYAVGDIL
EDKPELTPVAIQSGKLLAQRLFGASLEKCDYINVPTTVFTPLEYGCCGLSEEKAIEVYKK
ENLEIYHTLFWPLEWTVAGRENNTCYAKIICNKFDHDRVIGFHILGPNAGEVTQGFAAAM
KCGLTKQLLDDTIGIHPTCGEVFTTLEITKSSGLDITQKGCG
|
|
|
|---|
| BDBM50384998 |
|---|
| n/a |
|---|
| Name | BDBM50384998 |
|---|
| Synonyms: | CVD-0019905 | XANTHOHUMOL |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H22O5 |
|---|
| Mol. Mass. | 354.3964 |
|---|
| SMILES | [#6]-[#8]-c1cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c(-[#8])c1-[#6](=O)\[#6]=[#6]\c1ccc(-[#8])cc1 |
|---|
| Structure | 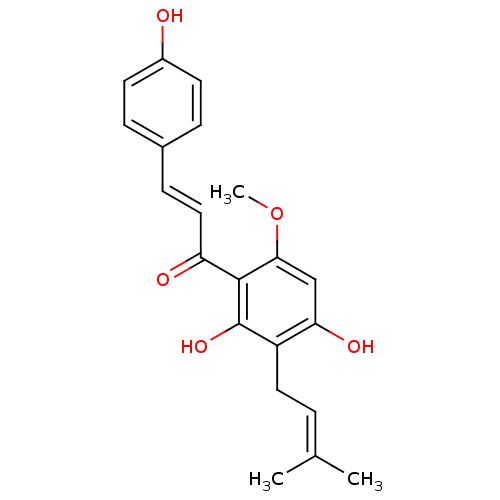
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhang, B; Duan, D; Ge, C; Yao, J; Liu, Y; Li, X; Fang, J Synthesis of xanthohumol analogues and discovery of potent thioredoxin reductase inhibitor as potential anticancer agent. J Med Chem58:1795-805 (2015) [PubMed] Article
Zhang, B; Duan, D; Ge, C; Yao, J; Liu, Y; Li, X; Fang, J Synthesis of xanthohumol analogues and discovery of potent thioredoxin reductase inhibitor as potential anticancer agent. J Med Chem58:1795-805 (2015) [PubMed] Article