

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Ephrin type-A receptor 3 | ||
| Ligand | BDBM50100316 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1454051 (CHEMBL3361797) | ||
| Kd | 8.6±n/a nM | ||
| Citation |  Unzue, A; Dong, J; Lafleur, K; Zhao, H; Frugier, E; Caflisch, A; Nevado, C Pyrrolo[3,2-b]quinoxaline derivatives as types I1/2 and II Eph tyrosine kinase inhibitors: structure-based design, synthesis, and in vivo validation. J Med Chem57:6834-44 (2014) [PubMed] Article Unzue, A; Dong, J; Lafleur, K; Zhao, H; Frugier, E; Caflisch, A; Nevado, C Pyrrolo[3,2-b]quinoxaline derivatives as types I1/2 and II Eph tyrosine kinase inhibitors: structure-based design, synthesis, and in vivo validation. J Med Chem57:6834-44 (2014) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Ephrin type-A receptor 3 | |||
| Name: | Ephrin type-A receptor 3 | ||
| Synonyms: | EPHA3 | EPHA3_HUMAN | ETK | ETK1 | Ephrin receptor | Ephrin type-A receptor 3 | Ephrin type-A receptor 3 (EPHA3) | HEK | TYRO4 | ||
| Type: | Protein | ||
| Mol. Mass.: | 110131.95 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P29320 | ||
| Residue: | 983 | ||
| Sequence: |
| ||
| BDBM50100316 | |||
| n/a | |||
| Name | BDBM50100316 | ||
| Synonyms: | CHEMBL3321809 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C18H15N5O2 | ||
| Mol. Mass. | 333.344 | ||
| SMILES | Cc1ccc(O)cc1-n1c(N)c(C(N)=O)c2nc3ccccc3nc12 |(9.45,-34.75,;10.95,-35.07,;11.43,-36.54,;12.94,-36.86,;13.97,-35.71,;15.47,-36.03,;13.49,-34.25,;11.98,-33.93,;11.51,-32.46,;12.41,-31.22,;13.95,-31.22,;11.51,-29.97,;11.98,-28.51,;13.47,-28.11,;10.95,-27.36,;10.04,-30.45,;8.71,-29.68,;7.38,-30.45,;6.04,-29.68,;4.71,-30.45,;4.71,-31.99,;6.04,-32.76,;7.38,-31.99,;8.71,-32.76,;10.04,-31.99,)| | ||
| Structure | 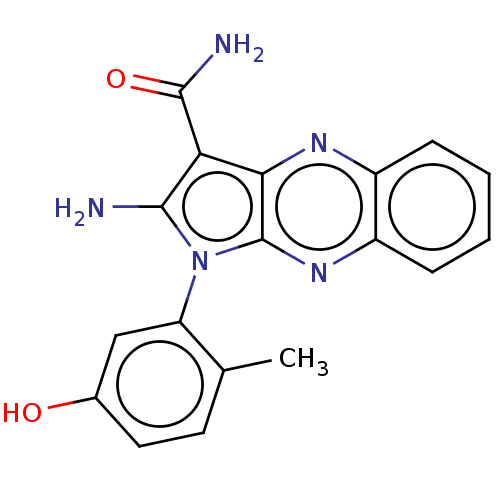
| ||