

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Mitogen-activated protein kinase kinase kinase kinase 4 | ||
| Ligand | BDBM50122725 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1519919 (CHEMBL3624922) | ||
| IC50 | 41±n/a nM | ||
| Citation |  Ndubaku, CO; Crawford, TD; Chen, H; Boggs, JW; Drobnick, J; Harris, SF; Jesudason, R; McNamara, E; Nonomiya, J; Sambrone, A; Schmidt, S; Smyczek, T; Vitorino, P; Wang, L; Wu, P; Yeung, S; Chen, J; Chen, K; Ding, CZ; Wang, T; Xu, Z; Gould, SE; Murray, LJ; Ye, W Structure-Based Design of GNE-495, a Potent and Selective MAP4K4 Inhibitor with Efficacy in Retinal Angiogenesis. ACS Med Chem Lett6:913-8 (2015) [PubMed] Article Ndubaku, CO; Crawford, TD; Chen, H; Boggs, JW; Drobnick, J; Harris, SF; Jesudason, R; McNamara, E; Nonomiya, J; Sambrone, A; Schmidt, S; Smyczek, T; Vitorino, P; Wang, L; Wu, P; Yeung, S; Chen, J; Chen, K; Ding, CZ; Wang, T; Xu, Z; Gould, SE; Murray, LJ; Ye, W Structure-Based Design of GNE-495, a Potent and Selective MAP4K4 Inhibitor with Efficacy in Retinal Angiogenesis. ACS Med Chem Lett6:913-8 (2015) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Mitogen-activated protein kinase kinase kinase kinase 4 | |||
| Name: | Mitogen-activated protein kinase kinase kinase kinase 4 | ||
| Synonyms: | HGK | HPK/GCK-like kinase HGK | KIAA0687 | M4K4_HUMAN | MAP4K4 | MAP4K4 (HGK) | MAPK/ERK kinase kinase kinase 4 | MEK kinase kinase 4 | MEKKK 4 | Mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4) | NIK | Nck-interacting kinase | ||
| Type: | Serine/threonine-protein kinase | ||
| Mol. Mass.: | 142114.73 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | O95819 | ||
| Residue: | 1239 | ||
| Sequence: |
| ||
| BDBM50122725 | |||
| n/a | |||
| Name | BDBM50122725 | ||
| Synonyms: | CHEMBL3623131 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H18FN3O2 | ||
| Mol. Mass. | 351.3742 | ||
| SMILES | Nc1ncc(C(=O)NC2CC(O)C2)c2ccc(cc12)-c1cccc(F)c1 |(-1.33,2.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;-1.33,-3.08,;-.26,-3.69,;-2.66,-3.85,;-2.65,-5.4,;-3.73,-6.44,;-2.64,-7.52,;-2.63,-8.75,;-1.55,-6.43,;,-.77,;1.33,-1.54,;2.66,-.77,;2.66,.77,;1.33,1.54,;,.77,;4,1.54,;5.33,.77,;6.66,1.53,;6.67,3.07,;5.33,3.85,;5.34,5.08,;4,3.08,)| | ||
| Structure | 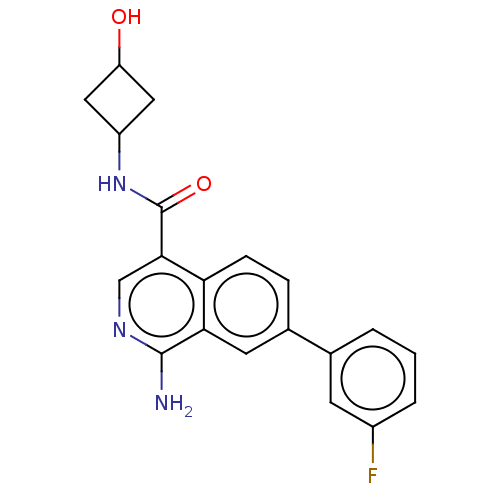
| ||