| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Kallikrein-14 |
|---|
| Ligand | BDBM50125021 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1522073 (CHEMBL3627278) |
|---|
| Ki | 1.2±n/a nM |
|---|
| Citation |  de Veer, SJ; Wang, CK; Harris, JM; Craik, DJ; Swedberg, JE Improving the Selectivity of Engineered Protease Inhibitors: Optimizing the P2 Prime Residue Using a Versatile Cyclic Peptide Library. J Med Chem58:8257-68 (2015) [PubMed] Article de Veer, SJ; Wang, CK; Harris, JM; Craik, DJ; Swedberg, JE Improving the Selectivity of Engineered Protease Inhibitors: Optimizing the P2 Prime Residue Using a Versatile Cyclic Peptide Library. J Med Chem58:8257-68 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Kallikrein-14 |
|---|
| Name: | Kallikrein-14 |
|---|
| Synonyms: | KLK-L6 | KLK14 | KLK14_HUMAN | KLKL6 | Kallikrein 14 | Kallikrein-14 | Kallikrein-like protein 6 | hK14 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 29136.99 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1282452 |
|---|
| Residue: | 267 |
|---|
| Sequence: | MSLRVLGSGTWPSAPKMFLLLTALQVLAIAMTQSQEDENKIIGGHTCTRSSQPWQAALLA
GPRRRFLCGGALLSGQWVITAAHCGRPILQVALGKHNLRRWEATQQVLRVVRQVTHPNYN
SRTHDNDLMLLQLQQPARIGRAVRPIEVTQACASPGTSCRVSGWGTISSPIARYPASLQC
VNINISPDEVCQKAYPRTITPGMVCAGVPQGGKDSCQGDSGGPLVCRGQLQGLVSWGMER
CALPGYPGVYTNLCKYRSWIEETMRDK
|
|
|
|---|
| BDBM50125021 |
|---|
| n/a |
|---|
| Name | BDBM50125021 |
|---|
| Synonyms: | CHEMBL3623779 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C58H92N20O20S2 |
|---|
| Mol. Mass. | 1453.603 |
|---|
| SMILES | [H][C@@]12CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| |
|---|
| Structure | 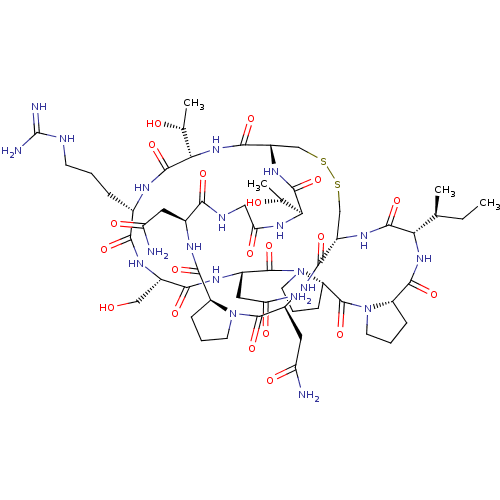
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 de Veer, SJ; Wang, CK; Harris, JM; Craik, DJ; Swedberg, JE Improving the Selectivity of Engineered Protease Inhibitors: Optimizing the P2 Prime Residue Using a Versatile Cyclic Peptide Library. J Med Chem58:8257-68 (2015) [PubMed] Article
de Veer, SJ; Wang, CK; Harris, JM; Craik, DJ; Swedberg, JE Improving the Selectivity of Engineered Protease Inhibitors: Optimizing the P2 Prime Residue Using a Versatile Cyclic Peptide Library. J Med Chem58:8257-68 (2015) [PubMed] Article