| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein phosphatase S |
|---|
| Ligand | BDBM50131835 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1526440 (CHEMBL3637709) |
|---|
| IC50 | 7900±n/a nM |
|---|
| Citation |  Haftchenary, S; Jouk, AO; Aubry, I; Lewis, AM; Landry, M; Ball, DP; Shouksmith, AE; Collins, CV; Tremblay, ML; Gunning, PT Identification of Bidentate Salicylic Acid Inhibitors of PTP1B. ACS Med Chem Lett6:982-6 (2015) [PubMed] Article Haftchenary, S; Jouk, AO; Aubry, I; Lewis, AM; Landry, M; Ball, DP; Shouksmith, AE; Collins, CV; Tremblay, ML; Gunning, PT Identification of Bidentate Salicylic Acid Inhibitors of PTP1B. ACS Med Chem Lett6:982-6 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein phosphatase S |
|---|
| Name: | Receptor-type tyrosine-protein phosphatase S |
|---|
| Synonyms: | PTPRS | PTPRS_HUMAN | R-PTP-S | R-PTP-sigma | Receptor-type tyrosine-protein phosphatase S | Receptor-type tyrosine-protein phosphatase sigma |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 217036.26 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_107614 |
|---|
| Residue: | 1948 |
|---|
| Sequence: | MAPTWGPGMVSVVGPMGLLVVLLVGGCAAEEPPRFIKEPKDQIGVSGGVASFVCQATGDP
KPRVTWNKKGKKVNSQRFETIEFDESAGAVLRIQPLRTPRDENVYECVAQNSVGEITVHA
KLTVLREDQLPSGFPNIDMGPQLKVVERTRTATMLCAASGNPDPEITWFKDFLPVDPSAS
NGRIKQLRSETFESTPIRGALQIESSEETDQGKYECVATNSAGVRYSSPANLYVRELREV
RRVAPRFSILPMSHEIMPGGNVNITCVAVGSPMPYVKWMQGAEDLTPEDDMPVGRNVLEL
TDVKDSANYTCVAMSSLGVIEAVAQITVKSLPKAPGTPMVTENTATSITITWDSGNPDPV
SYYVIEYKSKSQDGPYQIKEDITTTRYSIGGLSPNSEYEIWVSAVNSIGQGPPSESVVTR
TGEQAPASAPRNVQARMLSATTMIVQWEEPVEPNGLIRGYRVYYTMEPEHPVGNWQKHNV
DDSLLTTVGSLLEDETYTVRVLAFTSVGDGPLSDPIQVKTQQGVPGQPMNLRAEARSETS
ITLSWSPPRQESIIKYELLFREGDHGREVGRTFDPTTSYVVEDLKPNTEYAFRLAARSPQ
GLGAFTPVVRQRTLQSKPSAPPQDVKCVSVRSTAILVSWRPPPPETHNGALVGYSVRYRP
LGSEDPEPKEVNGIPPTTTQILLEALEKWTQYRITTVAHTEVGPGPESSPVVVRTDEDVP
SAPPRKVEAEALNATAIRVLWRSPAPGRQHGQIRGYQVHYVRMEGAEARGPPRIKDVMLA
DAQWETDDTAEYEMVITNLQPETAYSITVAAYTMKGDGARSKPKVVVTKGAVLGRPTLSV
QQTPEGSLLARWEPPAGTAEDQVLGYRLQFGREDSTPLATLEFPPSEDRYTASGVHKGAT
YVFRLAARSRGGLGEEAAEVLSIPEDTPRGHPQILEAAGNASAGTVLLRWLPPVPAERNG
AIVKYTVAVREAGALGPARETELPAAAEPGAENALTLQGLKPDTAYDLQVRAHTRRGPGP
FSPPVRYRTFLRDQVSPKNFKVKMIMKTSVLLSWEFPDNYNSPTPYKIQYNGLTLDVDGR
TTKKLITHLKPHTFYNFVLTNRGSSLGGLQQTVTAWTAFNLLNGKPSVAPKPDADGFIMV
YLPDGQSPVPVQSYFIVMVPLRKSRGGQFLTPLGSPEDMDLEELIQDISRLQRRSLRHSR
QLEVPRPYIAARFSVLPPTFHPGDQKQYGGFDNRGLEPGHRYVLFVLAVLQKSEPTFAAS
PFSDPFQLDNPDPQPIVDGEEGLIWVIGPVLAVVFIICIVIAILLYKNKPDSKRKDSEPR
TKCLLNNADLAPHHPKDPVEMRRINFQTPDSGLRSPLREPGFHFESMLSHPPIPIADMAE
HTERLKANDSLKLSQEYESIDPGQQFTWEHSNLEVNKPKNRYANVIAYDHSRVILQPIEG
IMGSDYINANYVDGYRCQNAYIATQGPLPETFGDFWRMVWEQRSATIVMMTRLEEKSRIK
CDQYWPNRGTETYGFIQVTLLDTIELATFCVRTFSLHKNGSSEKREVRQFQFTAWPDHGV
PEYPTPFLAFLRRVKTCNPPDAGPIVVHCSAGVGRTGCFIVIDAMLERIKPEKTVDVYGH
VTLMRSQRNYMVQTEDQYSFIHEALLEAVGCGNTEVPARSLYAYIQKLAQVEPGEHVTGM
ELEFKRLANSKAHTSRFISANLPCNKFKNRLVNIMPYESTRVCLQPIRGVEGSDYINASF
IDGYRQQKAYIATQGPLAETTEDFWRMLWENNSTIVVMLTKLREMGREKCHQYWPAERSA
RYQYFVVDPMAEYNMPQYILREFKVTDARDGQSRTVRQFQFTDWPEQGVPKSGEGFIDFI
GQVHKTKEQFGQDGPISVHCSAGVGRTGVFITLSIVLERMRYEGVVDIFQTVKMLRTQRP
AMVQTEDEYQFCYQAALEYLGSFDHYAT
|
|
|
|---|
| BDBM50131835 |
|---|
| n/a |
|---|
| Name | BDBM50131835 |
|---|
| Synonyms: | CHEMBL3632914 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C45H45N7O10S |
|---|
| Mol. Mass. | 875.945 |
|---|
| SMILES | OC(=O)c1ccc(NC(=O)CCCn2cc(CN(CC(=O)N(Cc3ccc(cc3)C3CCCCC3)c3ccc(C(O)=O)c(O)c3)S(=O)(=O)c3cccc4cccnc34)nn2)cc1O |
|---|
| Structure | 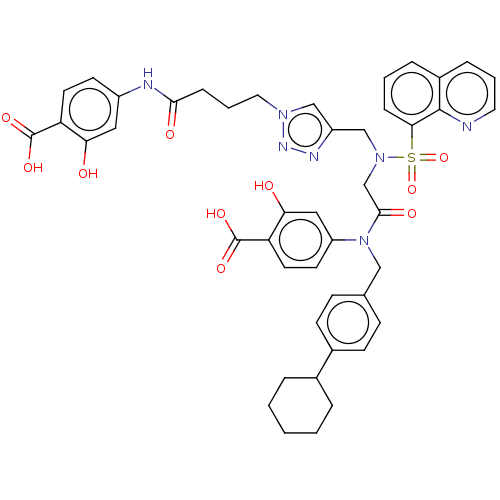
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Haftchenary, S; Jouk, AO; Aubry, I; Lewis, AM; Landry, M; Ball, DP; Shouksmith, AE; Collins, CV; Tremblay, ML; Gunning, PT Identification of Bidentate Salicylic Acid Inhibitors of PTP1B. ACS Med Chem Lett6:982-6 (2015) [PubMed] Article
Haftchenary, S; Jouk, AO; Aubry, I; Lewis, AM; Landry, M; Ball, DP; Shouksmith, AE; Collins, CV; Tremblay, ML; Gunning, PT Identification of Bidentate Salicylic Acid Inhibitors of PTP1B. ACS Med Chem Lett6:982-6 (2015) [PubMed] Article