| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2B |
|---|
| Ligand | BDBM50148861 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1557255 (CHEMBL3773630) |
|---|
| EC50 | 4460±n/a nM |
|---|
| Citation |  Cheng, J; Giguere, PM; Schmerberg, CM; Pogorelov, VM; Rodriguiz, RM; Huang, XP; Zhu, H; McCorvy, JD; Wetsel, WC; Roth, BL; Kozikowski, AP Further Advances in Optimizing (2-Phenylcyclopropyl)methylamines as Novel Serotonin 2C Agonists: Effects on Hyperlocomotion, Prepulse Inhibition, and Cognition Models. J Med Chem59:578-91 (2016) [PubMed] Article Cheng, J; Giguere, PM; Schmerberg, CM; Pogorelov, VM; Rodriguiz, RM; Huang, XP; Zhu, H; McCorvy, JD; Wetsel, WC; Roth, BL; Kozikowski, AP Further Advances in Optimizing (2-Phenylcyclopropyl)methylamines as Novel Serotonin 2C Agonists: Effects on Hyperlocomotion, Prepulse Inhibition, and Cognition Models. J Med Chem59:578-91 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2B |
|---|
| Name: | 5-hydroxytryptamine receptor 2B |
|---|
| Synonyms: | 5-HT-2B | 5-HT2B | 5-hydroxytryptamine (serotonin) receptor 2B [Homo sapiens] | 5-hydroxytryptamine receptor 2B (5-HT2B) | 5-hydroxytryptamine receptor 2C (5HT2C) | 5HT2B_HUMAN | HTR2B | Serotonin (5-HT3) receptor | Serotonin 2b (5-HT2b) receptor | Serotonin Receptor 2B |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 54312.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Receptor binding assays were performed using human clone stably expressed in CHO cells. |
|---|
| Residue: | 481 |
|---|
| Sequence: | MALSYRVSELQSTIPEHILQSTFVHVISSNWSGLQTESIPEEMKQIVEEQGNKLHWAALL
ILMVIIPTIGGNTLVILAVSLEKKLQYATNYFLMSLAVADLLVGLFVMPIALLTIMFEAM
WPLPLVLCPAWLFLDVLFSTASIMHLCAISVDRYIAIKKPIQANQYNSRATAFIKITVVW
LISIGIAIPVPIKGIETDVDNPNNITCVLTKERFGDFMLFGSLAAFFTPLAIMIVTYFLT
IHALQKKAYLVKNKPPQRLTWLTVSTVFQRDETPCSSPEKVAMLDGSRKDKALPNSGDET
LMRRTSTIGKKSVQTISNEQRASKVLGIVFFLFLLMWCPFFITNITLVLCDSCNQTTLQM
LLEIFVWIGYVSSGVNPLVYTLFNKTFRDAFGRYITCNYRATKSVKTLRKRSSKIYFRNP
MAENSKFFKKHGIRNGINPAMYQSPMRLRSSTIQSSSIILLDTLLLTENEGDKTEEQVSY
V
|
|
|
|---|
| BDBM50148861 |
|---|
| n/a |
|---|
| Name | BDBM50148861 |
|---|
| Synonyms: | CHEMBL3769499 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H16ClF2NO |
|---|
| Mol. Mass. | 275.722 |
|---|
| SMILES | Cl.NCC1CC1c1c(F)c(F)ccc1OCC=C |
|---|
| Structure | 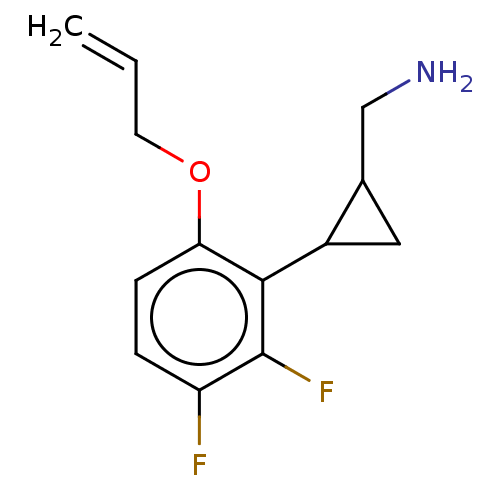
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cheng, J; Giguere, PM; Schmerberg, CM; Pogorelov, VM; Rodriguiz, RM; Huang, XP; Zhu, H; McCorvy, JD; Wetsel, WC; Roth, BL; Kozikowski, AP Further Advances in Optimizing (2-Phenylcyclopropyl)methylamines as Novel Serotonin 2C Agonists: Effects on Hyperlocomotion, Prepulse Inhibition, and Cognition Models. J Med Chem59:578-91 (2016) [PubMed] Article
Cheng, J; Giguere, PM; Schmerberg, CM; Pogorelov, VM; Rodriguiz, RM; Huang, XP; Zhu, H; McCorvy, JD; Wetsel, WC; Roth, BL; Kozikowski, AP Further Advances in Optimizing (2-Phenylcyclopropyl)methylamines as Novel Serotonin 2C Agonists: Effects on Hyperlocomotion, Prepulse Inhibition, and Cognition Models. J Med Chem59:578-91 (2016) [PubMed] Article