| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM162872 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1571195 (CHEMBL3795956) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Walsh, SP; Shahripour, A; Tang, H; de Jesus, RK; Teumelsan, N; Zhu, Y; Frie, J; Priest, BT; Swensen, AM; Alonso-Galicia, M; Felix, JP; Brochu, RM; Bailey, T; Thomas-Fowlkes, B; Zhou, X; Pai, LY; Hampton, C; Hernandez, M; Owens, K; Ehrhart, J; Roy, S; Kaczorowski, GJ; Yang, L; Garcia, ML; Pasternak, A Differentiation of ROMK potency from hERG potency in the phenacetyl piperazine series through heterocycle incorporation. Bioorg Med Chem Lett26:2339-43 (2016) [PubMed] Article Walsh, SP; Shahripour, A; Tang, H; de Jesus, RK; Teumelsan, N; Zhu, Y; Frie, J; Priest, BT; Swensen, AM; Alonso-Galicia, M; Felix, JP; Brochu, RM; Bailey, T; Thomas-Fowlkes, B; Zhou, X; Pai, LY; Hampton, C; Hernandez, M; Owens, K; Ehrhart, J; Roy, S; Kaczorowski, GJ; Yang, L; Garcia, ML; Pasternak, A Differentiation of ROMK potency from hERG potency in the phenacetyl piperazine series through heterocycle incorporation. Bioorg Med Chem Lett26:2339-43 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM162872 |
|---|
| n/a |
|---|
| Name | BDBM162872 |
|---|
| Synonyms: | US9056859, 72 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H25N7O3 |
|---|
| Mol. Mass. | 447.4897 |
|---|
| SMILES | Cc1c2COC(=O)c2ccc1CCN1CCN(CC1)C(=O)Cc1ccc(nc1)-n1cnnn1 |
|---|
| Structure | 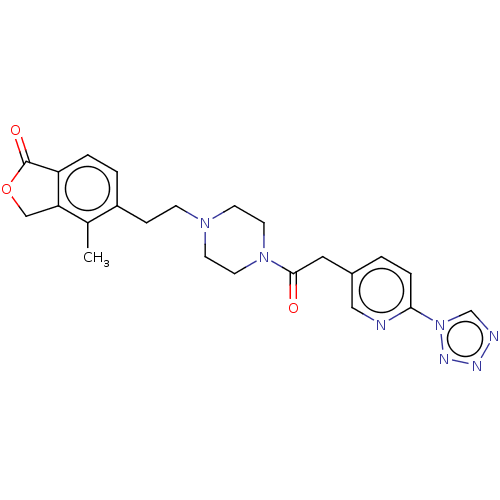
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Walsh, SP; Shahripour, A; Tang, H; de Jesus, RK; Teumelsan, N; Zhu, Y; Frie, J; Priest, BT; Swensen, AM; Alonso-Galicia, M; Felix, JP; Brochu, RM; Bailey, T; Thomas-Fowlkes, B; Zhou, X; Pai, LY; Hampton, C; Hernandez, M; Owens, K; Ehrhart, J; Roy, S; Kaczorowski, GJ; Yang, L; Garcia, ML; Pasternak, A Differentiation of ROMK potency from hERG potency in the phenacetyl piperazine series through heterocycle incorporation. Bioorg Med Chem Lett26:2339-43 (2016) [PubMed] Article
Walsh, SP; Shahripour, A; Tang, H; de Jesus, RK; Teumelsan, N; Zhu, Y; Frie, J; Priest, BT; Swensen, AM; Alonso-Galicia, M; Felix, JP; Brochu, RM; Bailey, T; Thomas-Fowlkes, B; Zhou, X; Pai, LY; Hampton, C; Hernandez, M; Owens, K; Ehrhart, J; Roy, S; Kaczorowski, GJ; Yang, L; Garcia, ML; Pasternak, A Differentiation of ROMK potency from hERG potency in the phenacetyl piperazine series through heterocycle incorporation. Bioorg Med Chem Lett26:2339-43 (2016) [PubMed] Article