| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50196096 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1618211 (CHEMBL3860380) |
|---|
| IC50 | 16700±n/a nM |
|---|
| Citation |  Lelais, G; Epple, R; Marsilje, TH; Long, YO; McNeill, M; Chen, B; Lu, W; Anumolu, J; Badiger, S; Bursulaya, B; DiDonato, M; Fong, R; Juarez, J; Li, J; Manuia, M; Mason, DE; Gordon, P; Groessl, T; Johnson, K; Jia, Y; Kasibhatla, S; Li, C; Isbell, J; Spraggon, G; Bender, S; Michellys, PY Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide (EGF816), a Novel, Potent, and WT Sparing Covalent Inhibitor of Oncogenic (L858R, ex19del) and Resistant (T790M) EGFR Mutants for the Treatment of EGFR Mutant Non-Small-Ce J Med Chem59:6671-89 (2016) [PubMed] Article Lelais, G; Epple, R; Marsilje, TH; Long, YO; McNeill, M; Chen, B; Lu, W; Anumolu, J; Badiger, S; Bursulaya, B; DiDonato, M; Fong, R; Juarez, J; Li, J; Manuia, M; Mason, DE; Gordon, P; Groessl, T; Johnson, K; Jia, Y; Kasibhatla, S; Li, C; Isbell, J; Spraggon, G; Bender, S; Michellys, PY Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide (EGF816), a Novel, Potent, and WT Sparing Covalent Inhibitor of Oncogenic (L858R, ex19del) and Resistant (T790M) EGFR Mutants for the Treatment of EGFR Mutant Non-Small-Ce J Med Chem59:6671-89 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50196096 |
|---|
| n/a |
|---|
| Name | BDBM50196096 |
|---|
| Synonyms: | CHEMBL3951434 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H32F3N5O2 |
|---|
| Mol. Mass. | 527.5812 |
|---|
| SMILES | CN(C)C\C=C\C(=O)N1CCCC[C@H](C1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cccc(C)c12 |r| |
|---|
| Structure | 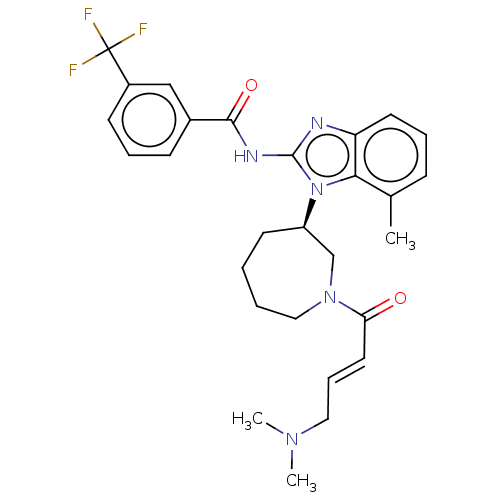
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lelais, G; Epple, R; Marsilje, TH; Long, YO; McNeill, M; Chen, B; Lu, W; Anumolu, J; Badiger, S; Bursulaya, B; DiDonato, M; Fong, R; Juarez, J; Li, J; Manuia, M; Mason, DE; Gordon, P; Groessl, T; Johnson, K; Jia, Y; Kasibhatla, S; Li, C; Isbell, J; Spraggon, G; Bender, S; Michellys, PY Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide (EGF816), a Novel, Potent, and WT Sparing Covalent Inhibitor of Oncogenic (L858R, ex19del) and Resistant (T790M) EGFR Mutants for the Treatment of EGFR Mutant Non-Small-Ce J Med Chem59:6671-89 (2016) [PubMed] Article
Lelais, G; Epple, R; Marsilje, TH; Long, YO; McNeill, M; Chen, B; Lu, W; Anumolu, J; Badiger, S; Bursulaya, B; DiDonato, M; Fong, R; Juarez, J; Li, J; Manuia, M; Mason, DE; Gordon, P; Groessl, T; Johnson, K; Jia, Y; Kasibhatla, S; Li, C; Isbell, J; Spraggon, G; Bender, S; Michellys, PY Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide (EGF816), a Novel, Potent, and WT Sparing Covalent Inhibitor of Oncogenic (L858R, ex19del) and Resistant (T790M) EGFR Mutants for the Treatment of EGFR Mutant Non-Small-Ce J Med Chem59:6671-89 (2016) [PubMed] Article