| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-X-C chemokine receptor type 3 |
|---|
| Ligand | BDBM50198921 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1621030 (CHEMBL3863313) |
|---|
| IC50 | <10000±n/a nM |
|---|
| Citation |  Abeywardane, A; Caviness, G; Choi, Y; Cogan, D; Gao, A; Goldberg, D; Heim-Riether, A; Jeanfavre, D; Klein, E; Kowalski, JA; Mao, W; Miller, C; Moss, N; Ramsden, P; Raymond, E; Skow, D; Smith-Keenan, L; Snow, RJ; Wu, F; Wu, JP; Yu, Y N-Arylsulfonyl-a-amino carboxamides are potent and selective inhibitors of the chemokine receptor CCR10 that show efficacy in the murine DNFB model of contact hypersensitivity. Bioorg Med Chem Lett26:5277-5283 (2016) [PubMed] Article Abeywardane, A; Caviness, G; Choi, Y; Cogan, D; Gao, A; Goldberg, D; Heim-Riether, A; Jeanfavre, D; Klein, E; Kowalski, JA; Mao, W; Miller, C; Moss, N; Ramsden, P; Raymond, E; Skow, D; Smith-Keenan, L; Snow, RJ; Wu, F; Wu, JP; Yu, Y N-Arylsulfonyl-a-amino carboxamides are potent and selective inhibitors of the chemokine receptor CCR10 that show efficacy in the murine DNFB model of contact hypersensitivity. Bioorg Med Chem Lett26:5277-5283 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-X-C chemokine receptor type 3 |
|---|
| Name: | C-X-C chemokine receptor type 3 |
|---|
| Synonyms: | AAO92295.1 | C-X-C chemokine receptor type 3 | C-X-C chemokine receptor type 3 (CXCR3) | C-X-C chemokine receptor type 3 (CXCR3A) | CXCR3 | CXCR3A | CXCR3_HUMAN | GPR9 | chemokine (C-X-C motif) receptor 3 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 40665.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 368 |
|---|
| Sequence: | MVLEVSDHQVLNDAEVAALLENFSSSYDYGENESDSCCTSPPCPQDFSLNFDRAFLPALY
SLLFLLGLLGNGAVAAVLLSRRTALSSTDTFLLHLAVADTLLVLTLPLWAVDAAVQWVFG
SGLCKVAGALFNINFYAGALLLACISFDRYLNIVHATQLYRRGPPARVTLTCLAVWGLCL
LFALPDFIFLSAHHDERLNATHCQYNFPQVGRTALRVLQLVAGFLLPLLVMAYCYAHILA
VLLVSRGQRRLRAMRLVVVVVVAFALCWTPYHLVVLVDILMDLGALARNCGRESRVDVAK
SVTSGLGYMHCCLNPLLYAFVGVKFRERMWMLLLRLGCPNQRGLQRQPSSSRRDSSWSET
SEASYSGL
|
|
|
|---|
| BDBM50198921 |
|---|
| n/a |
|---|
| Name | BDBM50198921 |
|---|
| Synonyms: | CHEMBL3889627 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H27N5O3S |
|---|
| Mol. Mass. | 453.557 |
|---|
| SMILES | CC1CCN(CC1)C(=O)C(CCn1cccc1C#N)NS(=O)(=O)c1cccc2[nH]ccc12 |
|---|
| Structure | 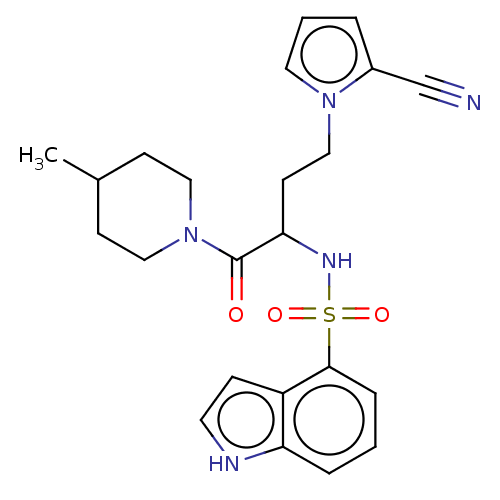
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Abeywardane, A; Caviness, G; Choi, Y; Cogan, D; Gao, A; Goldberg, D; Heim-Riether, A; Jeanfavre, D; Klein, E; Kowalski, JA; Mao, W; Miller, C; Moss, N; Ramsden, P; Raymond, E; Skow, D; Smith-Keenan, L; Snow, RJ; Wu, F; Wu, JP; Yu, Y N-Arylsulfonyl-a-amino carboxamides are potent and selective inhibitors of the chemokine receptor CCR10 that show efficacy in the murine DNFB model of contact hypersensitivity. Bioorg Med Chem Lett26:5277-5283 (2016) [PubMed] Article
Abeywardane, A; Caviness, G; Choi, Y; Cogan, D; Gao, A; Goldberg, D; Heim-Riether, A; Jeanfavre, D; Klein, E; Kowalski, JA; Mao, W; Miller, C; Moss, N; Ramsden, P; Raymond, E; Skow, D; Smith-Keenan, L; Snow, RJ; Wu, F; Wu, JP; Yu, Y N-Arylsulfonyl-a-amino carboxamides are potent and selective inhibitors of the chemokine receptor CCR10 that show efficacy in the murine DNFB model of contact hypersensitivity. Bioorg Med Chem Lett26:5277-5283 (2016) [PubMed] Article