| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-1A adrenergic receptor |
|---|
| Ligand | BDBM50160152 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_33887 (CHEMBL643759) |
|---|
| Ki | 1.3±n/a nM |
|---|
| Citation |  Lopez, FJ; Arias, L; Chan, R; Clarke, DE; Elworthy, TR; Ford, AP; Guzman, A; Jaime-Figueroa, S; Jasper, JR; Morgans, DJ; Padilla, F; Perez-Medrano, A; Quintero, C; Romero, M; Sandoval, L; Smith, SA; Williams, TJ; Blue, DR Synthesis, pharmacology and pharmacokinetics of 3-(4-aryl-piperazin-1-ylalkyl)-uracils as uroselective alpha1A-antagonists. Bioorg Med Chem Lett13:1873-8 (2003) [PubMed] Lopez, FJ; Arias, L; Chan, R; Clarke, DE; Elworthy, TR; Ford, AP; Guzman, A; Jaime-Figueroa, S; Jasper, JR; Morgans, DJ; Padilla, F; Perez-Medrano, A; Quintero, C; Romero, M; Sandoval, L; Smith, SA; Williams, TJ; Blue, DR Synthesis, pharmacology and pharmacokinetics of 3-(4-aryl-piperazin-1-ylalkyl)-uracils as uroselective alpha1A-antagonists. Bioorg Med Chem Lett13:1873-8 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-1A adrenergic receptor |
|---|
| Name: | Alpha-1A adrenergic receptor |
|---|
| Synonyms: | ADA1A_HUMAN | ADRA1A | ADRA1C | Adrenergic alpha1A | Alpha 1A-adrenoceptor | Alpha 1A-adrenoreceptor | Alpha adrenergic receptor 1a | Alpha-1C adrenergic receptor | Alpha-adrenergic receptor 1c | Cerebral cortex alpha adrenergic receptor | adrenergic Alpha1 | adrenergic Alpha1C |
|---|
| Type: | Cell-surface receptors |
|---|
| Mol. Mass.: | 51511.67 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35348 |
|---|
| Residue: | 466 |
|---|
| Sequence: | MVFLSGNASDSSNCTQPPAPVNISKAILLGVILGGLILFGVLGNILVILSVACHRHLHSV
THYYIVNLAVADLLLTSTVLPFSAIFEVLGYWAFGRVFCNIWAAVDVLCCTASIMGLCII
SIDRYIGVSYPLRYPTIVTQRRGLMALLCVWALSLVISIGPLFGWRQPAPEDETICQINE
EPGYVLFSALGSFYLPLAIILVMYCRVYVVAKRESRGLKSGLKTDKSDSEQVTLRIHRKN
APAGGSGMASAKTKTHFSVRLLKFSREKKAAKTLGIVVGCFVLCWLPFFLVMPIGSFFPD
FKPSETVFKIVFWLGYLNSCINPIIYPCSSQEFKKAFQNVLRIQCLCRKQSSKHALGYTL
HPPSQAVEGQHKDMVRIPVGSRETFYRISKTDGVCEWKFFSSMPRGSARITVSKDQSSCT
TARVRSKSFLQVCCCVGPSTPSLDKNHQVPTIKVHTISLSENGEEV
|
|
|
|---|
| BDBM50160152 |
|---|
| n/a |
|---|
| Name | BDBM50160152 |
|---|
| Synonyms: | 3-(3-{3-[4-Fluoro-2-(2,2,2-trifluoro-ethoxy)-phenyl]-piperazin-1-yl}-propyl)-5-methyl-1H-pyrimidine-2,4-dione | 3-(3-{4-[4-Fluoro-2-(2,2,2-trifluoro-ethoxy)-phenyl]-piperazin-1-yl}-propyl)-5-methyl-1H-pyrimidine-2,4-dione | CHEMBL24777 | RS-100975 | Ro-700004 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H24F4N4O3 |
|---|
| Mol. Mass. | 444.4232 |
|---|
| SMILES | Cc1c[nH]c(=O)n(CCCN2CCN(CC2)c2ccc(F)cc2OCC(F)(F)F)c1=O |
|---|
| Structure | 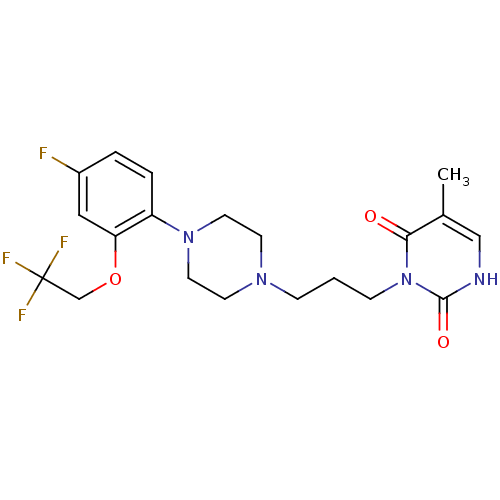
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lopez, FJ; Arias, L; Chan, R; Clarke, DE; Elworthy, TR; Ford, AP; Guzman, A; Jaime-Figueroa, S; Jasper, JR; Morgans, DJ; Padilla, F; Perez-Medrano, A; Quintero, C; Romero, M; Sandoval, L; Smith, SA; Williams, TJ; Blue, DR Synthesis, pharmacology and pharmacokinetics of 3-(4-aryl-piperazin-1-ylalkyl)-uracils as uroselective alpha1A-antagonists. Bioorg Med Chem Lett13:1873-8 (2003) [PubMed]
Lopez, FJ; Arias, L; Chan, R; Clarke, DE; Elworthy, TR; Ford, AP; Guzman, A; Jaime-Figueroa, S; Jasper, JR; Morgans, DJ; Padilla, F; Perez-Medrano, A; Quintero, C; Romero, M; Sandoval, L; Smith, SA; Williams, TJ; Blue, DR Synthesis, pharmacology and pharmacokinetics of 3-(4-aryl-piperazin-1-ylalkyl)-uracils as uroselective alpha1A-antagonists. Bioorg Med Chem Lett13:1873-8 (2003) [PubMed]