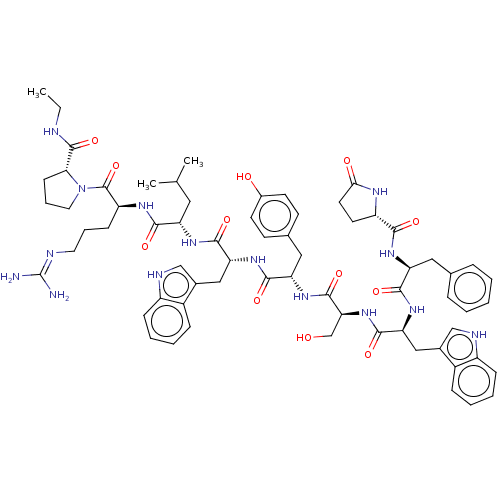
| SMILES | CCNC(=O)[C@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC(=O)N1 |wU:45.57,31.44,63.79,23.28,5.4,88.94,wD:57.63,77.91,12.20,(27.09,-8.95,;27.47,-10.44,;28.96,-10.86,;30.04,-11.96,;31.53,-11.6,;29.61,-13.45,;30.56,-14.67,;29.69,-15.95,;28.21,-15.53,;28.16,-13.97,;26.88,-13.11,;26.98,-11.58,;25.49,-13.78,;25.38,-15.33,;26.66,-16.2,;26.55,-17.73,;27.83,-18.59,;27.72,-20.13,;29,-21,;26.33,-20.8,;24.22,-12.92,;22.82,-13.6,;22.72,-15.13,;21.54,-12.73,;21.65,-11.2,;23.05,-10.52,;24.32,-11.38,;23.15,-8.98,;20.15,-13.41,;18.89,-12.55,;18.99,-11.01,;17.49,-13.23,;17.39,-14.76,;18.14,-16.08,;19.64,-16.43,;19.78,-17.94,;18.38,-18.56,;17.91,-20.01,;16.43,-20.33,;15.39,-19.17,;15.88,-17.73,;17.36,-17.42,;16.22,-12.36,;14.83,-13.03,;14.71,-14.57,;13.55,-12.17,;13.66,-10.62,;15.04,-9.95,;15.16,-8.42,;16.54,-7.74,;17.82,-8.6,;19.2,-7.93,;17.71,-10.14,;16.32,-10.81,;12.16,-12.85,;10.88,-11.97,;10.99,-10.44,;9.49,-12.65,;9.39,-14.19,;10.67,-15.06,;8.21,-11.79,;6.83,-12.46,;6.71,-14.01,;5.54,-11.6,;5.65,-10.07,;7.05,-9.39,;8.58,-9.18,;8.85,-7.67,;7.49,-6.93,;7.12,-5.44,;5.63,-5,;4.53,-6.07,;4.89,-7.56,;6.37,-8,;4.17,-12.27,;2.88,-11.41,;3,-9.87,;1.49,-12.09,;1.38,-13.62,;2.15,-14.95,;3.66,-14.95,;4.41,-16.27,;3.65,-17.59,;2.12,-17.59,;1.37,-16.26,;.22,-11.23,;-1.18,-11.9,;-1.28,-13.44,;-2.34,-11.11,;-2.34,-9.69,;-3.87,-9.46,;-4.55,-10.83,;-6.06,-11.09,;-3.45,-11.92,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Haviy, F; Fitzpatrick, TD; Nichols, CJ; Swenson, RE; Bush, EN; Diaz, G; Nguyen, A; Nellans, HN; Hoffman, DJ; Ghanbari, H Stabilization of the N-terminal residues of luteinizing hormone-releasing hormone agonists and the effect on pharmacokinetics. J Med Chem35:3890-4 (1992) [PubMed]
Haviy, F; Fitzpatrick, TD; Nichols, CJ; Swenson, RE; Bush, EN; Diaz, G; Nguyen, A; Nellans, HN; Hoffman, DJ; Ghanbari, H Stabilization of the N-terminal residues of luteinizing hormone-releasing hormone agonists and the effect on pharmacokinetics. J Med Chem35:3890-4 (1992) [PubMed]