

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Neuronal acetylcholine receptor subunit alpha-7 | ||
| Ligand | BDBM50211215 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEBML_1648183 | ||
| EC50 | 110±n/a nM | ||
| Citation |  Iwuagwu, C; King, D; McDonald, IM; Cook, J; Zusi, FC; Hill, MD; Mate, RA; Fang, H; Knox, R; Gallagher, L; Post-Munson Amy Easton, D; Miller, R; Benitex, Y; Siuciak, J; Lodge, N; Zaczek, R; Morgan, D; Bristow, L; Macor, JE; Olson, RE Design and synthesis of a novel series of 4-heteroarylamino-1'-azaspiro[oxazole-5,3'-bicyclo[2.2.2]octanes asa7 nicotinic receptor agonists 2. Development of 4-heteroaryl SAR. Bioorg Med Chem Lett27:1261-1266 (2017) [PubMed] Article Iwuagwu, C; King, D; McDonald, IM; Cook, J; Zusi, FC; Hill, MD; Mate, RA; Fang, H; Knox, R; Gallagher, L; Post-Munson Amy Easton, D; Miller, R; Benitex, Y; Siuciak, J; Lodge, N; Zaczek, R; Morgan, D; Bristow, L; Macor, JE; Olson, RE Design and synthesis of a novel series of 4-heteroarylamino-1'-azaspiro[oxazole-5,3'-bicyclo[2.2.2]octanes asa7 nicotinic receptor agonists 2. Development of 4-heteroaryl SAR. Bioorg Med Chem Lett27:1261-1266 (2017) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Neuronal acetylcholine receptor subunit alpha-7 | |||
| Name: | Neuronal acetylcholine receptor subunit alpha-7 | ||
| Synonyms: | ACHA7_RAT | Acra7 | Cholinergic, Nicotinic Alpha7 | Cholinergic, Nicotinic Alpha7/5-HT3 | Chrna7 | Neuronal acetylcholine receptor | Neuronal acetylcholine receptor (alpha7 nAChR) | Neuronal acetylcholine receptor subunit alpha 7 | Neuronal acetylcholine receptor subunit alpha-7 | Neuronal acetylcholine receptor subunit alpha-7 (nAChR alpha7) | Neuronal acetylcholine receptor subunit alpha-7 (nAChR) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 56502.44 | ||
| Organism: | Rattus norvegicus (Rat) | ||
| Description: | Q05941 | ||
| Residue: | 502 | ||
| Sequence: |
| ||
| BDBM50211215 | |||
| n/a | |||
| Name | BDBM50211215 | ||
| Synonyms: | CHEMBL3898922 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C14H18N4O | ||
| Mol. Mass. | 258.3189 | ||
| SMILES | C1N=C(Nc2ccccn2)O[C@]11CN2CCC1CC2 |r,wU:11.11,t:1,THB:10:11:14.15:18.17,(46.07,-8.61,;46.9,-7.32,;45.93,-6.12,;46.33,-4.64,;47.81,-4.23,;48.2,-2.75,;49.68,-2.35,;50.78,-3.44,;50.38,-4.92,;48.9,-5.33,;44.5,-6.68,;44.58,-8.22,;44.88,-9.74,;43.39,-9.05,;41.73,-9.76,;41.51,-8.26,;43.11,-7.57,;43.19,-5.78,;43.67,-6.99,)| | ||
| Structure | 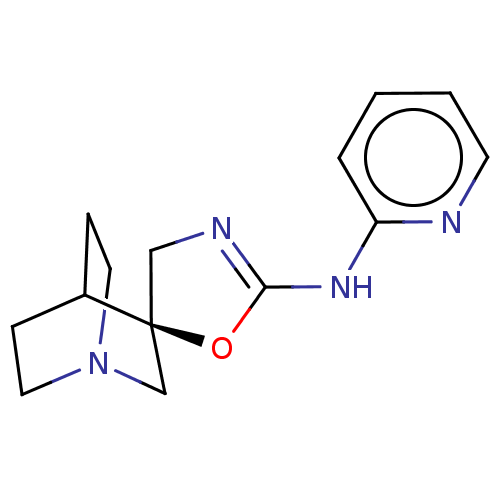
| ||