| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Kappa-type opioid receptor |
|---|
| Ligand | BDBM50236880 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1659540 (CHEMBL4009152) |
|---|
| EC50 | 4.5±n/a nM |
|---|
| Citation |  Soeberdt, M; Molenveld, P; Storcken, RP; Bouzanne des Mazery, R; Sterk, GJ; Autar, R; Bolster, MG; Wagner, C; Aerts, SN; van Holst, FR; Wegert, A; Tangherlini, G; Frehland, B; Schepmann, D; Metze, D; Lotts, T; Knie, U; Lin, KY; Huang, TY; Lai, CC; Ständer, S; Wünsch, B; Abels, C Design and Synthesis of Enantiomerically Pure Decahydroquinoxalines as Potent and Selective¿-Opioid Receptor Agonists with Anti-Inflammatory Activity in Vivo. J Med Chem60:2526-2551 (2017) [PubMed] Article Soeberdt, M; Molenveld, P; Storcken, RP; Bouzanne des Mazery, R; Sterk, GJ; Autar, R; Bolster, MG; Wagner, C; Aerts, SN; van Holst, FR; Wegert, A; Tangherlini, G; Frehland, B; Schepmann, D; Metze, D; Lotts, T; Knie, U; Lin, KY; Huang, TY; Lai, CC; Ständer, S; Wünsch, B; Abels, C Design and Synthesis of Enantiomerically Pure Decahydroquinoxalines as Potent and Selective¿-Opioid Receptor Agonists with Anti-Inflammatory Activity in Vivo. J Med Chem60:2526-2551 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Kappa-type opioid receptor |
|---|
| Name: | Kappa-type opioid receptor |
|---|
| Synonyms: | K-OR-1 | KOR-1 | Kappa-opioid receptor (KOR) | Kappa-type opioid receptor (KOPR) | Kappa-type opioid receptor (KOR) | Kappa-type opioid receptor (Kappa) | OPIATE Kappa | OPRK | OPRK1 | OPRK_HUMAN | kappa opioid receptor (KOR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 42648.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P41145 |
|---|
| Residue: | 380 |
|---|
| Sequence: | MDSPIQIFRGEPGPTCAPSACLPPNSSAWFPGWAEPDSNGSAGSEDAQLEPAHISPAIPV
IITAVYSVVFVVGLVGNSLVMFVIIRYTKMKTATNIYIFNLALADALVTTTMPFQSTVYL
MNSWPFGDVLCKIVISIDYYNMFTSIFTLTMMSVDRYIAVCHPVKALDFRTPLKAKIINI
CIWLLSSSVGISAIVLGGTKVREDVDVIECSLQFPDDDYSWWDLFMKICVFIFAFVIPVL
IIIVCYTLMILRLKSVRLLSGSREKDRNLRRITRLVLVVVAVFVVCWTPIHIFILVEALG
STSHSTAALSSYYFCIALGYTNSSLNPILYAFLDENFKRCFRDFCFPLKMRMERQSTSRV
RNTVQDPAYLRDIDGMNKPV
|
|
|
|---|
| BDBM50236880 |
|---|
| n/a |
|---|
| Name | BDBM50236880 |
|---|
| Synonyms: | CHEMBL4102311 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H34Cl2N4O2 |
|---|
| Mol. Mass. | 481.458 |
|---|
| SMILES | [H][C@]12CCC[C@H](N3CCCC3)[C@@]1([H])N(CCN2C(=O)NC(C)C)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| |
|---|
| Structure | 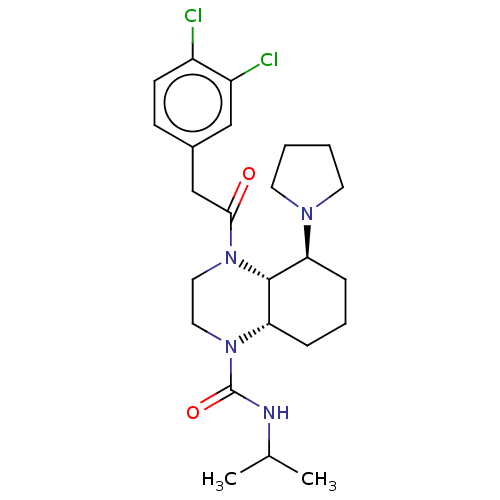
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Soeberdt, M; Molenveld, P; Storcken, RP; Bouzanne des Mazery, R; Sterk, GJ; Autar, R; Bolster, MG; Wagner, C; Aerts, SN; van Holst, FR; Wegert, A; Tangherlini, G; Frehland, B; Schepmann, D; Metze, D; Lotts, T; Knie, U; Lin, KY; Huang, TY; Lai, CC; Ständer, S; Wünsch, B; Abels, C Design and Synthesis of Enantiomerically Pure Decahydroquinoxalines as Potent and Selective¿-Opioid Receptor Agonists with Anti-Inflammatory Activity in Vivo. J Med Chem60:2526-2551 (2017) [PubMed] Article
Soeberdt, M; Molenveld, P; Storcken, RP; Bouzanne des Mazery, R; Sterk, GJ; Autar, R; Bolster, MG; Wagner, C; Aerts, SN; van Holst, FR; Wegert, A; Tangherlini, G; Frehland, B; Schepmann, D; Metze, D; Lotts, T; Knie, U; Lin, KY; Huang, TY; Lai, CC; Ständer, S; Wünsch, B; Abels, C Design and Synthesis of Enantiomerically Pure Decahydroquinoxalines as Potent and Selective¿-Opioid Receptor Agonists with Anti-Inflammatory Activity in Vivo. J Med Chem60:2526-2551 (2017) [PubMed] Article