| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50014323 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1659857 (CHEMBL4009469) |
|---|
| IC50 | 12±n/a nM |
|---|
| Citation |  Horley, NJ; Beresford, KJ; Chawla, T; McCann, GJ; Ruparelia, KC; Gatchie, L; Sonawane, VR; Williams, IS; Tan, HL; Joshi, P; Bharate, SS; Kumar, V; Bharate, SB; Chaudhuri, B Discovery and characterization of novel CYP1B1 inhibitors based on heterocyclic chalcones: Overcoming cisplatin resistance in CYP1B1-overexpressing lines. Eur J Med Chem129:159-174 (2017) [PubMed] Article Horley, NJ; Beresford, KJ; Chawla, T; McCann, GJ; Ruparelia, KC; Gatchie, L; Sonawane, VR; Williams, IS; Tan, HL; Joshi, P; Bharate, SS; Kumar, V; Bharate, SB; Chaudhuri, B Discovery and characterization of novel CYP1B1 inhibitors based on heterocyclic chalcones: Overcoming cisplatin resistance in CYP1B1-overexpressing lines. Eur J Med Chem129:159-174 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50014323 |
|---|
| n/a |
|---|
| Name | BDBM50014323 |
|---|
| Synonyms: | 2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo[h]chromen-4-one | 7,8-Benzoflavone | CHEMBL283196 | alpha-naphthoflavone |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H12O2 |
|---|
| Mol. Mass. | 272.2974 |
|---|
| SMILES | O=c1cc(oc2c3ccccc3ccc12)-c1ccccc1 |
|---|
| Structure | 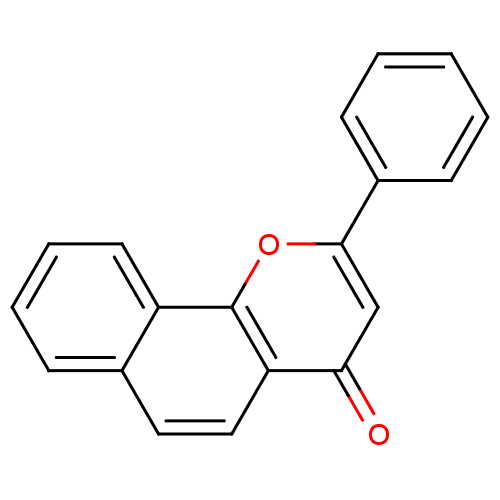
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Horley, NJ; Beresford, KJ; Chawla, T; McCann, GJ; Ruparelia, KC; Gatchie, L; Sonawane, VR; Williams, IS; Tan, HL; Joshi, P; Bharate, SS; Kumar, V; Bharate, SB; Chaudhuri, B Discovery and characterization of novel CYP1B1 inhibitors based on heterocyclic chalcones: Overcoming cisplatin resistance in CYP1B1-overexpressing lines. Eur J Med Chem129:159-174 (2017) [PubMed] Article
Horley, NJ; Beresford, KJ; Chawla, T; McCann, GJ; Ruparelia, KC; Gatchie, L; Sonawane, VR; Williams, IS; Tan, HL; Joshi, P; Bharate, SS; Kumar, V; Bharate, SB; Chaudhuri, B Discovery and characterization of novel CYP1B1 inhibitors based on heterocyclic chalcones: Overcoming cisplatin resistance in CYP1B1-overexpressing lines. Eur J Med Chem129:159-174 (2017) [PubMed] Article