| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50237514 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1660136 (CHEMBL4009748) |
|---|
| IC50 | 20000±n/a nM |
|---|
| Citation |  Wu, YJ; Guernon, J; Shi, J; Ditta, J; Robbins, KJ; Rajamani, R; Easton, A; Newton, A; Bourin, C; Mosure, K; Soars, MG; Knox, RJ; Matchett, M; Pieschl, RL; Post-Munson, DJ; Wang, S; Herrington, J; Graef, J; Newberry, K; Bristow, LJ; Meanwell, NA; Olson, R; Thompson, LA; Dzierba, C Development of New Benzenesulfonamides As Potent and Selective Na J Med Chem60:2513-2525 (2017) [PubMed] Article Wu, YJ; Guernon, J; Shi, J; Ditta, J; Robbins, KJ; Rajamani, R; Easton, A; Newton, A; Bourin, C; Mosure, K; Soars, MG; Knox, RJ; Matchett, M; Pieschl, RL; Post-Munson, DJ; Wang, S; Herrington, J; Graef, J; Newberry, K; Bristow, LJ; Meanwell, NA; Olson, R; Thompson, LA; Dzierba, C Development of New Benzenesulfonamides As Potent and Selective Na J Med Chem60:2513-2525 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50237514 |
|---|
| n/a |
|---|
| Name | BDBM50237514 |
|---|
| Synonyms: | CHEMBL4089010 | US10836758, Example 6 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H16ClFN4O3S2 |
|---|
| Mol. Mass. | 406.883 |
|---|
| SMILES | Fc1cc(NCC2CNCCO2)c(Cl)cc1S(=O)(=O)Nc1nccs1 |
|---|
| Structure | 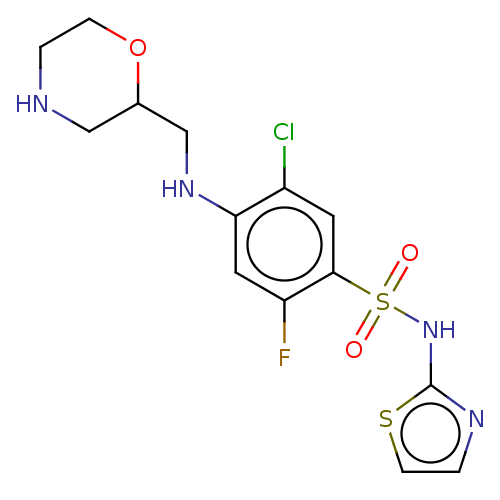
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wu, YJ; Guernon, J; Shi, J; Ditta, J; Robbins, KJ; Rajamani, R; Easton, A; Newton, A; Bourin, C; Mosure, K; Soars, MG; Knox, RJ; Matchett, M; Pieschl, RL; Post-Munson, DJ; Wang, S; Herrington, J; Graef, J; Newberry, K; Bristow, LJ; Meanwell, NA; Olson, R; Thompson, LA; Dzierba, C Development of New Benzenesulfonamides As Potent and Selective Na J Med Chem60:2513-2525 (2017) [PubMed] Article
Wu, YJ; Guernon, J; Shi, J; Ditta, J; Robbins, KJ; Rajamani, R; Easton, A; Newton, A; Bourin, C; Mosure, K; Soars, MG; Knox, RJ; Matchett, M; Pieschl, RL; Post-Munson, DJ; Wang, S; Herrington, J; Graef, J; Newberry, K; Bristow, LJ; Meanwell, NA; Olson, R; Thompson, LA; Dzierba, C Development of New Benzenesulfonamides As Potent and Selective Na J Med Chem60:2513-2525 (2017) [PubMed] Article