| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuropeptide Y receptor type 2 |
|---|
| Ligand | BDBM82286 |
|---|
| Substrate/Competitor | n/a |
|---|
| Ki | 340±n/a nM |
|---|
| Comments | PDSP_1511 |
|---|
| Citation |  Matsumoto, M; Nomura, T; Momose, K; Ikeda, Y; Kondou, Y; Akiho, H; Togami, J; Kimura, Y; Okada, M; Yamaguchi, T Inactivation of a novel neuropeptide Y/peptide YY receptor gene in primate species. J Biol Chem271:27217-20 (1996) [PubMed] Article Matsumoto, M; Nomura, T; Momose, K; Ikeda, Y; Kondou, Y; Akiho, H; Togami, J; Kimura, Y; Okada, M; Yamaguchi, T Inactivation of a novel neuropeptide Y/peptide YY receptor gene in primate species. J Biol Chem271:27217-20 (1996) [PubMed] Article |
|---|
| More Info.: | Get all data from this article |
|---|
| |
| Neuropeptide Y receptor type 2 |
|---|
| Name: | Neuropeptide Y receptor type 2 |
|---|
| Synonyms: | NPY-Y2 | NPY2R | Neuropeptide Y receptor Y2 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 42919.43 |
|---|
| Organism: | RABBIT |
|---|
| Description: | B6VRS5 |
|---|
| Residue: | 382 |
|---|
| Sequence: | MGPINAEADENQTVEEMKMEPYGPGQPTPRGELAPDPEPELIDSTKLIEVQVVLILAYCS
IILLGVIGNSLVIHVVIKFKSMRTVTNFFIANLAVADLLVNTLCLPFTLTYTLMGEWKMG
PVLCHLVPYAQGLAVQVSTITLTVIALDRHRCIVYHLESKISKRISFLIIGLAWGISALL
ASPLAIFREYSLIEIIPDFEIVACTEKWPGEEKSIYSTIYSLSSLLILYVLPLGIISFSY
TRIWSKLKNHISPGTASDHYHQRRQKTTKMLVCVVVVFAVSWLPLHAFQLAVDIDSQVLD
LKEYKLIFTVFHIIAMCSTFANPLLYGWMNSNYRKAFLSAFRCEQRMDAIHSEVSVTFKA
KKNLEVKKNNGPHDSFTEATNV
|
|
|
|---|
| BDBM82286 |
|---|
| n/a |
|---|
| Name | BDBM82286 |
|---|
| Synonyms: | CAS_59763-91-6 | PP, human | PP,SALMON |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C185H289N53O54S2 |
|---|
| Mol. Mass. | 4183.726 |
|---|
| SMILES | [#6]-[#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7+]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#16]-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6](-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6](-[#6])-[#7])-[#6](-[#6])-[#6])-[#6](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O |
|---|
| Structure | 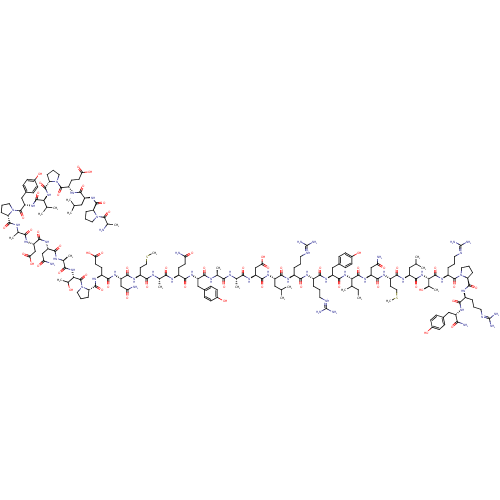
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Matsumoto, M; Nomura, T; Momose, K; Ikeda, Y; Kondou, Y; Akiho, H; Togami, J; Kimura, Y; Okada, M; Yamaguchi, T Inactivation of a novel neuropeptide Y/peptide YY receptor gene in primate species. J Biol Chem271:27217-20 (1996) [PubMed] Article
Matsumoto, M; Nomura, T; Momose, K; Ikeda, Y; Kondou, Y; Akiho, H; Togami, J; Kimura, Y; Okada, M; Yamaguchi, T Inactivation of a novel neuropeptide Y/peptide YY receptor gene in primate species. J Biol Chem271:27217-20 (1996) [PubMed] Article