| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1B |
|---|
| Ligand | BDBM85595 |
|---|
| Substrate/Competitor | n/a |
|---|
| Ki | 1000±n/a nM |
|---|
| Comments | PDSP_833 |
|---|
| Citation |  Millan, MJ; Newman-Tancredi, A; Audinot, V; Cussac, D; Lejeune, F; Nicolas, JP; Cogé, F; Galizzi, JP; Boutin, JA; Rivet, JM; Dekeyne, A; Gobert, A Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse35:79-95 (2000) [PubMed] Article Millan, MJ; Newman-Tancredi, A; Audinot, V; Cussac, D; Lejeune, F; Nicolas, JP; Cogé, F; Galizzi, JP; Boutin, JA; Rivet, JM; Dekeyne, A; Gobert, A Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse35:79-95 (2000) [PubMed] Article |
|---|
| More Info.: | Get all data from this article |
|---|
| |
| 5-hydroxytryptamine receptor 1B |
|---|
| Name: | 5-hydroxytryptamine receptor 1B |
|---|
| Synonyms: | 5-HT1B | 5HT1B_CAVPO | HTR1B |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 43125.84 |
|---|
| Organism: | GUINEA PIG |
|---|
| Description: | 5-HT1B HTR1B GUINEA PIG::O08892 |
|---|
| Residue: | 389 |
|---|
| Sequence: | MGNPEASCTPPAVLGSQTGLPHANVSAPPNNCSAPSHIYQDSIALPWKVLLVVLLALITL
ATTLSNAFVIATVYRTRKLHTPANYLIASLAFTDLLVSILVMPISTMYTVTGRWTLGQAL
CDFWLSSDITCCTASIMHLCVIALDRYWAITDAVGYSAKRTPRRAAGMIALVWVFSICIS
LPPFFWRQAKAEEEVLDCLVNTDHVLYTVYSTGGAFYLPTLLLIALYGRIYVEARSRILK
QTPNKTGKRLTRAQLITDSPGSTSSVTSINSRAPEVPCDSGSPVYVNQVKVRVSDALLEK
KKLMAARERKATKTLGVILGAFIVCWLPFFIISLVMPICKDACWFHMAIFDFFTWLGYLN
SLINPIIYTMSNEDFKQAFHKLIRFKCTT
|
|
|
|---|
| BDBM85595 |
|---|
| n/a |
|---|
| Name | BDBM85595 |
|---|
| Synonyms: | CAS_105182-45-4 | Fluparoxan | NSC_72036 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H10FNO2 |
|---|
| Mol. Mass. | 195.1903 |
|---|
| SMILES | Fc1cccc2OC3CNCC3Oc12 |
|---|
| Structure | 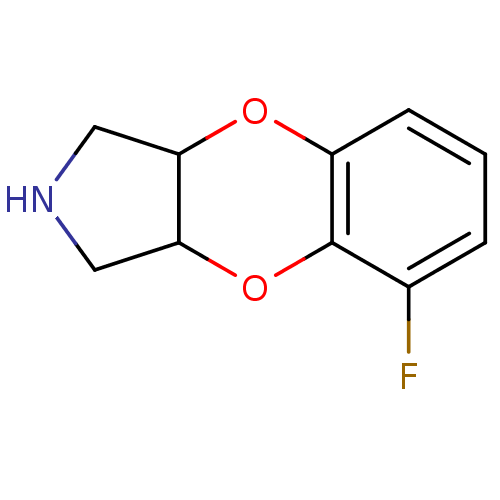
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Millan, MJ; Newman-Tancredi, A; Audinot, V; Cussac, D; Lejeune, F; Nicolas, JP; Cogé, F; Galizzi, JP; Boutin, JA; Rivet, JM; Dekeyne, A; Gobert, A Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse35:79-95 (2000) [PubMed] Article
Millan, MJ; Newman-Tancredi, A; Audinot, V; Cussac, D; Lejeune, F; Nicolas, JP; Cogé, F; Galizzi, JP; Boutin, JA; Rivet, JM; Dekeyne, A; Gobert, A Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse35:79-95 (2000) [PubMed] Article