| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Hypoxanthine-guanine phosphoribosyltransferase |
|---|
| Ligand | BDBM92357 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Inhbition Assay |
|---|
| pH | 7.6±0 |
|---|
| Temperature | 310.15±0 K |
|---|
| Ki | 4940±290 nM |
|---|
| Citation |  Hazleton, KZ; Ho, MC; Cassera, MB; Clinch, K; Crump, DR; Rosario, I; Merino, EF; Almo, SC; Tyler, PC; Schramm, VL Acyclic Immucillin Phosphonates: Second-Generation Inhibitors of Plasmodium falciparum Hypoxanthine- Guanine-Xanthine Phosphoribosyltransferase. Chem Biol19:721-30 (2012) [PubMed] Article Hazleton, KZ; Ho, MC; Cassera, MB; Clinch, K; Crump, DR; Rosario, I; Merino, EF; Almo, SC; Tyler, PC; Schramm, VL Acyclic Immucillin Phosphonates: Second-Generation Inhibitors of Plasmodium falciparum Hypoxanthine- Guanine-Xanthine Phosphoribosyltransferase. Chem Biol19:721-30 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Hypoxanthine-guanine phosphoribosyltransferase |
|---|
| Name: | Hypoxanthine-guanine phosphoribosyltransferase |
|---|
| Synonyms: | HGPRT | HGPRTase | HPRT | HPRT1 | HPRT_HUMAN | Hypoxanthine-guanine phosphoribosyltransferase | Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 24579.61 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00492 |
|---|
| Residue: | 218 |
|---|
| Sequence: | MATRSPGVVISDDEPGYDLDLFCIPNHYAEDLERVFIPHGLIMDRTERLARDVMKEMGGH
HIVALCVLKGGYKFFADLLDYIKALNRNSDRSIPMTVDFIRLKSYCNDQSTGDIKVIGGD
DLSTLTGKNVLIVEDIIDTGKTMQTLLSLVRQYNPKMVKVASLLVKRTPRSVGYKPDFVG
FEIPDKFVVGYALDYNEYFRDLNHVCVISETGKAKYKA
|
|
|
|---|
| BDBM92357 |
|---|
| n/a |
|---|
| Name | BDBM92357 |
|---|
| Synonyms: | HGXPRT Inhibitor, 2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C9H12N4O4P |
|---|
| Mol. Mass. | 271.1903 |
|---|
| SMILES | [O-]P([O-])(=O)CCC[NH2+]c1c[nH]c2c1nc[nH]c2=O |
|---|
| Structure | 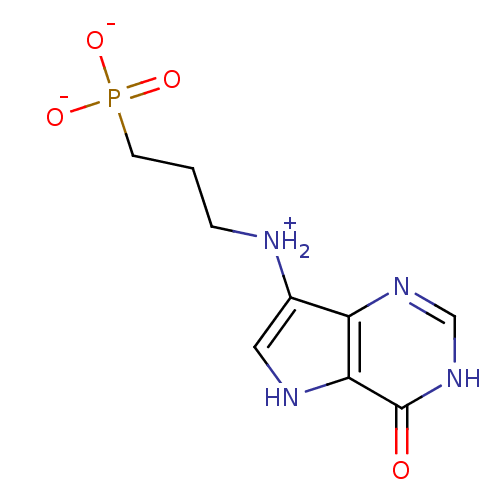
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hazleton, KZ; Ho, MC; Cassera, MB; Clinch, K; Crump, DR; Rosario, I; Merino, EF; Almo, SC; Tyler, PC; Schramm, VL Acyclic Immucillin Phosphonates: Second-Generation Inhibitors of Plasmodium falciparum Hypoxanthine- Guanine-Xanthine Phosphoribosyltransferase. Chem Biol19:721-30 (2012) [PubMed] Article
Hazleton, KZ; Ho, MC; Cassera, MB; Clinch, K; Crump, DR; Rosario, I; Merino, EF; Almo, SC; Tyler, PC; Schramm, VL Acyclic Immucillin Phosphonates: Second-Generation Inhibitors of Plasmodium falciparum Hypoxanthine- Guanine-Xanthine Phosphoribosyltransferase. Chem Biol19:721-30 (2012) [PubMed] Article