| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lysine-specific demethylase 5A |
|---|
| Ligand | BDBM50346863 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Biological Assay |
|---|
| pH | 7.5±n/a |
|---|
| Ki | 61000±n/a nM |
|---|
| Comments | extracted |
|---|
| Citation |  Minucci, S; Mai, A; Mattevi, A Tranylcypromine derivatives as inhibitors of histone demethylases LSD1 and/or LSD2 US Patent US8765820 Publication Date 7/1/2014 Minucci, S; Mai, A; Mattevi, A Tranylcypromine derivatives as inhibitors of histone demethylases LSD1 and/or LSD2 US Patent US8765820 Publication Date 7/1/2014 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lysine-specific demethylase 5A |
|---|
| Name: | Lysine-specific demethylase 5A |
|---|
| Synonyms: | Jarid1a | KDM5A_MOUSE | Kdm5a | Lysine-specific histone demethylase 2 (LSD2) | Rbp2 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 192210.15 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | Q3UXZ9 |
|---|
| Residue: | 1690 |
|---|
| Sequence: | MASVGPGGYAAEFVPPPECPVFEPSWEEFTDPLSFIGRIRPFAEKTGICKIRPPKDWQPP
FACEVKTFRFTPRVQRLNELEAMTRVRLDFLDQLAKFWELQGSTLKIPVVERKILDLYAL
SKIVASKGGFEIVTKEKKWSKVGSRLGYLPGKGTGSLLKSHYERILYPYELFQSGVSLMG
VQMPDLDLKEKVEAEVLSTDIQPSPERGTRMNIPPKRTRRVKSQSDSGEVNRNTELKKLQ
IFGAGPKVVGLAVGAKDKEDEVTRRRKVTNRSDAFNMQMRQRKGTLSVNFVDLYVCMFCG
RGNNEDKLLLCDGCDDSYHTFCLLPPLPDVPKGDWRCPKCVAEECNKPREAFGFEQAVRE
YTLQSFGEMADNFKSDYFNMPVHMVPTELVEKEFWRLVSSIEEDVIVEYGADISSKDFGS
GFPKKDGQRKMLPEEEEYALSGWNLNNMPVLEQSVLAHINVDISGMKVPWLYVGMCFSSF
CWHIEDHWSYSINYLHWGEPKTWYGVPSHAAEQLEEVMRELAPELFESQPDLLHQLVTIM
NPNVLMEHGVPVYRTNQCAGEFVVTFPRAYHSGFNQGYNFAEAVNFCTADWLPIGRQCVN
HYRRLRRHCVFSHEELIFKMAADPECLDVGLAAMVCKELTLMTEEETRLRESVVQMGVVM
SEEEVFELVPDDERQCSACRTTCFLSALTCSCNPERLVCLYHPTDLCSCPMQNKCLRYRY
PLEDLPSLLYGVKVRAQSYDTWVNRVTEALSASFNHKKDLIELRVMLEDAEDRKYPENDL
FRKLRDAVKEAETCGSVAQLLLSKKQKHRQSSDSGKTRTKLTVEELKAFVQQLVSLPCVI
SQTRQVKNLLDDVEEFHERAQEAMMDETPDSSKLQMLIDMGSSLYVELPELPRLKQELQQ
ARWLDEVRLTLSDPQQVTLDVMKKLIDSGVGLAPHHAVEKAMAELQELLTVSERWEEKAK
VCLQARPRHSMANLENIVNEAKNIPAFLPNVLSLKEALQKAREWTAKVEAIQSGNNYAYL
EQLESLSAKGRPIPVRLDALPQVESQVAAARAWRERTGRTFLKKNSSHTLLQVLSPRTDI
GVYGSGKNRRKKVKEIIEKEKEKDLDLEPLSDLEEGLEESRDTAMVVAVFKEREQKEIEA
MHSLRAANLAKMTIVERIEEVKFCICRKTASGFMLQCELCKDWFHNSCVPLPKSSSQKKG
SSWQAKDVKFLCPLCMRSRRPRLETILSLLVSLQKLPVRLPEGEALQCLTERAMSWQDKA
RQALATDELSSALAKLSVLSQRMVEQAAREKTEKIISAELQKAAANPDLQGHLPSFQQSA
FNRVVSSVSSSPHQTMDYDDEETDSDEDIRETYGYDMKDTASVKSSSSLEPNLFCDEEIP
IKSEEVVTHMWTAPSFCAEHAYSSASKSCSQGSSTPRKQPRKSPLVPRSLEPPVLELSPG
AKAQLEELMMVGDLLEVSLDETQHIWRILQATHPPSEDRFLHIMEDDSIEEKPLKMKGKD
SSEKKRKRKLEKVEQLFGEGKQKTKELKKIDKPKKKKLKLNVDKSKELNKLAKKLAKEEE
RKKKKEKAAAAKVELVKESTEKKRERKVLDIPSKYDWSGAEESDDENAVCAAQNCQRPCK
DKVDWVQCDGGCDEWFHQVCVGVSAEMAENEDYICINCAKKQGPDSPGQAPPPPFLMSYK
LPMEDLKETS
|
|
|
|---|
| BDBM50346863 |
|---|
| n/a |
|---|
| Name | BDBM50346863 |
|---|
| Synonyms: | CHEMBL1797640 | US8765820, 5b |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H16N2O |
|---|
| Mol. Mass. | 252.311 |
|---|
| SMILES | NC1CC1c1ccc(NC(=O)c2ccccc2)cc1 |
|---|
| Structure | 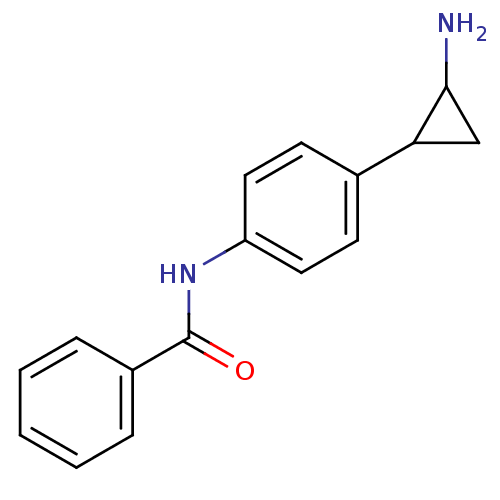
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Minucci, S; Mai, A; Mattevi, A Tranylcypromine derivatives as inhibitors of histone demethylases LSD1 and/or LSD2 US Patent US8765820 Publication Date 7/1/2014
Minucci, S; Mai, A; Mattevi, A Tranylcypromine derivatives as inhibitors of histone demethylases LSD1 and/or LSD2 US Patent US8765820 Publication Date 7/1/2014