

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | 5-hydroxytryptamine receptor 5A | ||
| Ligand | BDBM135787 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Binding Inhibition Assay | ||
| pH | 7.5±n/a | ||
| Temperature | 298.15±n/a K | ||
| Ki | 7.1±n/a nM | ||
| Comments | extracted | ||
| Citation |  Kinoyama, I; Miyazaki, T; Koganemaru, Y; Washio, T; Hamaguchi, W Nitrogenous-ring acylguanidine derivative US Patent US8853242 Publication Date 10/7/2014 Kinoyama, I; Miyazaki, T; Koganemaru, Y; Washio, T; Hamaguchi, W Nitrogenous-ring acylguanidine derivative US Patent US8853242 Publication Date 10/7/2014 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| 5-hydroxytryptamine receptor 5A | |||
| Name: | 5-hydroxytryptamine receptor 5A | ||
| Synonyms: | 5-HT-5 | 5-HT-5A | 5-hydroxytryptamine receptor 5 (5-HT5) | 5-hydroxytryptamine receptor 5A (5-HT5A) | 5HT5A_HUMAN | HTR5A | Serotonin (5-HT) receptor | Serotonin receptor 5A | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 40266.25 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P47898 | ||
| Residue: | 357 | ||
| Sequence: |
| ||
| BDBM135787 | |||
| n/a | |||
| Name | BDBM135787 | ||
| Synonyms: | US8853242, 159 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C18H14F2N4O2 | ||
| Mol. Mass. | 356.3262 | ||
| SMILES | COc1cccc(F)c1-c1ncc(F)c2ccc(cc12)C(=O)N=C(N)N |(-5.08,-2.13,;-5.85,-.8,;-5.08,.53,;-5.78,2,;-5,3.33,;-3.47,3.33,;-2.69,2,;-1.15,2,;-3.47,.67,;-2.69,-.67,;-3.47,-2,;-2.69,-3.33,;-1.15,-3.33,;-.38,-4.67,;-.38,-2,;1.15,-2,;1.92,-.67,;1.15,.67,;-.38,.67,;-1.15,-.67,;1.92,2,;1.15,3.33,;3.47,2,;4.23,3.33,;5.78,3.33,;3.47,4.67,)| | ||
| Structure | 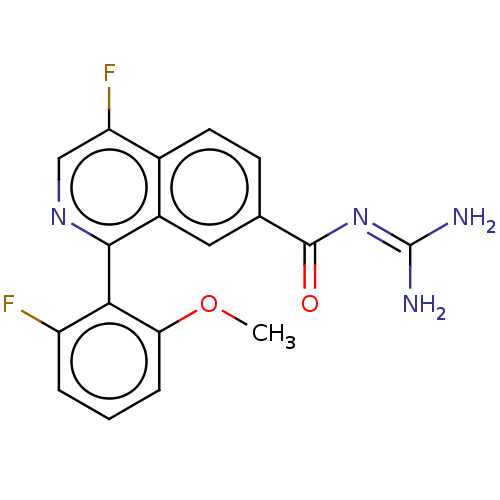
| ||