| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuraminidase |
|---|
| Ligand | BDBM4940 |
|---|
| Substrate/Competitor | BDBM4702 |
|---|
| Meas. Tech. | Neuraminidase Inhibition Assay |
|---|
| IC50 | 2±n/a nM |
|---|
| Citation |  Smith, PW; Sollis, SL; Howes, PD; Cherry, PC; Starkey, ID; Cobley, KN; Weston, H; Scicinski, J; Merritt, A; Whittington, A; Wyatt, P; Taylor, N; Green, D; Bethell, R; Madar, S; Fenton, RJ; Morley, PJ; Pateman, T; Beresford, A Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 1. Discovery, synthesis, biological activity, and structure-activity relationships of 4-guanidino- and 4-amino-4H-pyran-6-carboxamides. J Med Chem41:787-97 (1998) [PubMed] Article Smith, PW; Sollis, SL; Howes, PD; Cherry, PC; Starkey, ID; Cobley, KN; Weston, H; Scicinski, J; Merritt, A; Whittington, A; Wyatt, P; Taylor, N; Green, D; Bethell, R; Madar, S; Fenton, RJ; Morley, PJ; Pateman, T; Beresford, A Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 1. Discovery, synthesis, biological activity, and structure-activity relationships of 4-guanidino- and 4-amino-4H-pyran-6-carboxamides. J Med Chem41:787-97 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Neuraminidase |
|---|
| Name: | Neuraminidase |
|---|
| Synonyms: | Influenza A Virus Neuraminidase | NA | NRAM_I57A5 | Neuraminidase A |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52000.86 |
|---|
| Organism: | Influenza A virus (A/Singapore/1/57(H2N2)) |
|---|
| Description: | Q6XUE4 |
|---|
| Residue: | 469 |
|---|
| Sequence: | MNPNQKIITIGSVSLTIATVCFLMQIAILATTVTLHFKQHECDSPASNQVMPCEPIIIER
NITEIVYLNNTTIEKEICPEVVEYRNWSKPQCQITGFAPFSKDNSIRLSAGGDIWVTREP
YVSCDPGKCYQFALGQGTTLDNKHSNGTIHDRIPHRTLLMNELGVPFHLGTKQVCVAWSS
SSCHDGKAWLHVCVTGDDRNATASFIYDGRLVDSIGSWSQNILRTQESECVCINGTCTVV
MTDGSASGRADTRILFIKEGKIVHISPLSGSAQHIEECSCYPRYPDVRCICRDNWKGSNR
PVIDINMEDYSIDSSYVCSGLVGDTPRNDDSSSNSNCRDPNNERGNPGVKGWAFDNGDDV
WMGRTINKDSRSGYETFKVIGGWSTPNSKSQVNRQVIVDNNNWSGYSGIFSVEGKSCINR
CFYVELIRGRPQETRVWWTSNSIVVFCGTSGTYGTGSWPDGANINFMPI
|
|
|
|---|
| BDBM4940 |
|---|
| BDBM4702 |
|---|
| Name | BDBM4940 |
|---|
| Synonyms: | (2R,3R,4S)-4-carbamimidamido-2-(dipropylcarbamoyl)-3-acetamido-3,4-dihydro-2H-pyran-6-carboxylic acid | carboxamide deriv. 5g |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H27N5O5 |
|---|
| Mol. Mass. | 369.4161 |
|---|
| SMILES | [H][C@]1([#8]-[#6](=[#6]-[#6@H](\[#7]=[#6](/[#7])-[#7])-[#6@H]1-[#7]-[#6](-[#6])=O)-[#6](-[#8])=O)[#6](=O)-[#7](-[#6]-[#6]-[#6])-[#6]-[#6]-[#6] |r,c:3| |
|---|
| Structure | 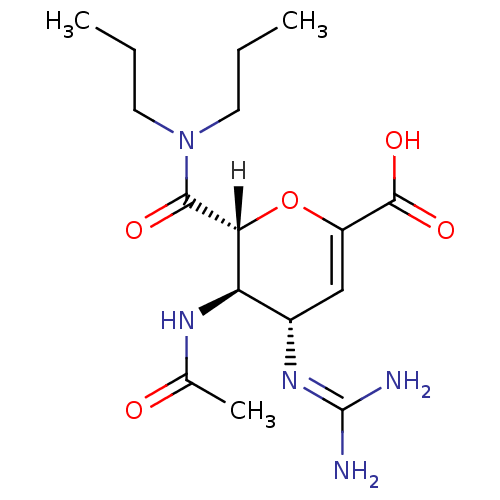
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Smith, PW; Sollis, SL; Howes, PD; Cherry, PC; Starkey, ID; Cobley, KN; Weston, H; Scicinski, J; Merritt, A; Whittington, A; Wyatt, P; Taylor, N; Green, D; Bethell, R; Madar, S; Fenton, RJ; Morley, PJ; Pateman, T; Beresford, A Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 1. Discovery, synthesis, biological activity, and structure-activity relationships of 4-guanidino- and 4-amino-4H-pyran-6-carboxamides. J Med Chem41:787-97 (1998) [PubMed] Article
Smith, PW; Sollis, SL; Howes, PD; Cherry, PC; Starkey, ID; Cobley, KN; Weston, H; Scicinski, J; Merritt, A; Whittington, A; Wyatt, P; Taylor, N; Green, D; Bethell, R; Madar, S; Fenton, RJ; Morley, PJ; Pateman, T; Beresford, A Dihydropyrancarboxamides related to zanamivir: a new series of inhibitors of influenza virus sialidases. 1. Discovery, synthesis, biological activity, and structure-activity relationships of 4-guanidino- and 4-amino-4H-pyran-6-carboxamides. J Med Chem41:787-97 (1998) [PubMed] Article