| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Ligand | BDBM50156669 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Inhibition of Steady-State Catalysis Assay |
|---|
| IC50 | 79.4±12 nM |
|---|
| Citation |  Leung, KK; Shilton, BH Quinone reductase 2 is an adventitious target of protein kinase CK2 inhibitors TBBz (TBI) and DMAT. Biochemistry54:47-59 (2015) [PubMed] Article Leung, KK; Shilton, BH Quinone reductase 2 is an adventitious target of protein kinase CK2 inhibitors TBBz (TBI) and DMAT. Biochemistry54:47-59 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Name: | Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Synonyms: | Metallothionein-3 | NMOR2 | NQO2 | NQO2_HUMAN | NRH dehydrogenase [quinone] 2 | NRH:quinone oxidoreductase 2 | QR2 | Quinone reductase 2 | Quinone reductase 2 (NQO2) | Ribosyldihydronicotinamide dehydrogenase [quinone] |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 25917.25 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P16083 |
|---|
| Residue: | 231 |
|---|
| Sequence: | MAGKKVLIVYAHQEPKSFNGSLKNVAVDELSRQGCTVTVSDLYAMNLEPRATDKDITGTL
SNPEVFNYGVETHEAYKQRSLASDITDEQKKVREADLVIFQFPLYWFSVPAILKGWMDRV
LCQGFAFDIPGFYDSGLLQGKLALLSVTTGGTAEMYTKTGVNGDSRYFLWPLQHGTLHFC
GFKVLAPQISFAPEIASEEERKGMVAAWSQRLQTIWKEEPIPCTAHWHFGQ
|
|
|
|---|
| BDBM50156669 |
|---|
| n/a |
|---|
| Name | BDBM50156669 |
|---|
| Synonyms: | 4,5,6,7-TETRABROMO-BENZIMIDAZOLE | 4,5,6,7-tetrabromo-1H-benzimidazole | 4,5,6,7-tetrabromo-1H-benzo[d]imidazole | 4,5,6,7-tetrabromo-1Hbenzimidazole (1) | 4,5,6,7-tetrabromobenzimidazole | CHEMBL373937 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C7H2Br4N2 |
|---|
| Mol. Mass. | 433.72 |
|---|
| SMILES | Brc1c(Br)c(Br)c2[nH]cnc2c1Br |
|---|
| Structure | 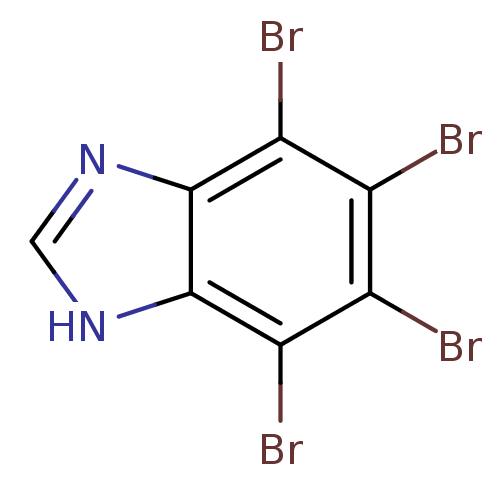
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Leung, KK; Shilton, BH Quinone reductase 2 is an adventitious target of protein kinase CK2 inhibitors TBBz (TBI) and DMAT. Biochemistry54:47-59 (2015) [PubMed] Article
Leung, KK; Shilton, BH Quinone reductase 2 is an adventitious target of protein kinase CK2 inhibitors TBBz (TBI) and DMAT. Biochemistry54:47-59 (2015) [PubMed] Article