| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuraminidase |
|---|
| Ligand | BDBM5024 |
|---|
| Substrate/Competitor | BDBM4702 |
|---|
| Meas. Tech. | Neuraminidase Inhibition Assay |
|---|
| pH | 6.5±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| IC50 | <1±n/a nM |
|---|
| Citation |  Chand, P; Kotian, PL; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Babu, YS; Bantia, S; Elliott, AJ; Montgomery, JA Systematic structure-based design and stereoselective synthesis of novel multisubstituted cyclopentane derivatives with potent antiinfluenza activity. J Med Chem44:4379-92 (2001) [PubMed] Article Chand, P; Kotian, PL; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Babu, YS; Bantia, S; Elliott, AJ; Montgomery, JA Systematic structure-based design and stereoselective synthesis of novel multisubstituted cyclopentane derivatives with potent antiinfluenza activity. J Med Chem44:4379-92 (2001) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Neuraminidase |
|---|
| Name: | Neuraminidase |
|---|
| Synonyms: | Influenza A Virus Neuraminidase | NA | NRAM_I57A5 | Neuraminidase A |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52000.86 |
|---|
| Organism: | Influenza A virus (A/Singapore/1/57(H2N2)) |
|---|
| Description: | Q6XUE4 |
|---|
| Residue: | 469 |
|---|
| Sequence: | MNPNQKIITIGSVSLTIATVCFLMQIAILATTVTLHFKQHECDSPASNQVMPCEPIIIER
NITEIVYLNNTTIEKEICPEVVEYRNWSKPQCQITGFAPFSKDNSIRLSAGGDIWVTREP
YVSCDPGKCYQFALGQGTTLDNKHSNGTIHDRIPHRTLLMNELGVPFHLGTKQVCVAWSS
SSCHDGKAWLHVCVTGDDRNATASFIYDGRLVDSIGSWSQNILRTQESECVCINGTCTVV
MTDGSASGRADTRILFIKEGKIVHISPLSGSAQHIEECSCYPRYPDVRCICRDNWKGSNR
PVIDINMEDYSIDSSYVCSGLVGDTPRNDDSSSNSNCRDPNNERGNPGVKGWAFDNGDDV
WMGRTINKDSRSGYETFKVIGGWSTPNSKSQVNRQVIVDNNNWSGYSGIFSVEGKSCINR
CFYVELIRGRPQETRVWWTSNSIVVFCGTSGTYGTGSWPDGANINFMPI
|
|
|
|---|
| BDBM5024 |
|---|
| BDBM4702 |
|---|
| Name | BDBM5024 |
|---|
| Synonyms: | (-)-(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylbutyl]-4-{[amino(imino)methyl]amino}-2-hydroxycyclopentanecarboxylic Acid | (1S,2S,3R,4R)-4-carbamimidamido-3-[(1S)-1-acetamido-2-ethylbutyl]-2-hydroxycyclopentane-1-carboxylic acid | BCX-1812 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H28N4O4 |
|---|
| Mol. Mass. | 328.4072 |
|---|
| SMILES | [H][C@](NC(C)=O)(C(CC)CC)[C@@H]1[C@H](O)[C@H](C[C@H]1N=C(N)N)C(O)=O |r| |
|---|
| Structure | 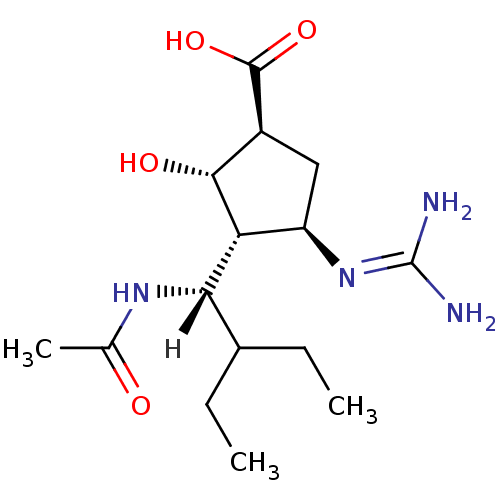
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chand, P; Kotian, PL; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Babu, YS; Bantia, S; Elliott, AJ; Montgomery, JA Systematic structure-based design and stereoselective synthesis of novel multisubstituted cyclopentane derivatives with potent antiinfluenza activity. J Med Chem44:4379-92 (2001) [PubMed] Article
Chand, P; Kotian, PL; Dehghani, A; El-Kattan, Y; Lin, TH; Hutchison, TL; Babu, YS; Bantia, S; Elliott, AJ; Montgomery, JA Systematic structure-based design and stereoselective synthesis of novel multisubstituted cyclopentane derivatives with potent antiinfluenza activity. J Med Chem44:4379-92 (2001) [PubMed] Article