

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Amyloid-beta precursor protein | ||
| Ligand | BDBM136833 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Release Assay | ||
| Temperature | 310.15±n/a K | ||
| IC50 | 1±n/a nM | ||
| Comments | extracted | ||
| Citation |  Karlstrom, S; Csjernyik, G; Swahn, B; Sandberg, L; Kolmodin, K; Soderman, P; Ohberg, L 2H-imidazol-4-amine compounds and their use as BACE inhibitors US Patent US9000182 Publication Date 4/7/2015 Karlstrom, S; Csjernyik, G; Swahn, B; Sandberg, L; Kolmodin, K; Soderman, P; Ohberg, L 2H-imidazol-4-amine compounds and their use as BACE inhibitors US Patent US9000182 Publication Date 4/7/2015 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Amyloid-beta precursor protein | |||
| Name: | Amyloid-beta precursor protein | ||
| Synonyms: | A4 | A4_HUMAN | ABPP | AD1 | AICD-50 | AICD-57 | AICD-59 | AID(50) | AID(57) | AID(59) | APP | APPI | Alzheimer disease amyloid protein | Amyloid beta A4 protein | Amyloid beta Protein | Amyloid beta protein (sAPPbeta) | Amyloid beta protein Abeta(1-42) | Amyloid intracellular domain 50 | Amyloid intracellular domain 57 | Amyloid intracellular domain 59 | Amyloid protein (Abeta42b) | Amyloid β-protein (Aβ42) | Beta amyloid A4 protein | Beta-APP40 | Beta-APP42 | Beta-amyloid protein 40 | Beta-amyloid protein 42 | C31 | C83 | C99 | CVAP | Cerebral vascular amyloid peptide | Gamma Secretase | Gamma-CTF(50) | Gamma-CTF(57) | Gamma-CTF(59) | Gamma-secretase | Gamma-secretase C-terminal fragment 50 | Gamma-secretase C-terminal fragment 57 | Gamma-secretase C-terminal fragment 59 | P3(40) | P3(42) | PN-II | PreA4 | Protease nexin-II | S-APP-alpha | S-APP-beta | Soluble APP-alpha | Soluble APP-beta | beta-Amyloid Precursor Protein (APP) | ||
| Type: | Single-pass type I membrane protein | ||
| Mol. Mass.: | 86890.41 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P05067 | ||
| Residue: | 770 | ||
| Sequence: |
| ||
| BDBM136833 | |||
| n/a | |||
| Name | BDBM136833 | ||
| Synonyms: | US10231967, Example 73 | US8865911, 73 | US9000182, 21, isomer 1 | US9918985, Example 73 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C25H26N4O | ||
| Mol. Mass. | 398.5001 | ||
| SMILES | CC#Cc1cncc(c1)-c1ccc2C[C@@]3(CC[C@H](O)CC3)[C@]3(N=C(C)C(N)=N3)c2c1 |r,wU:21.23,wD:14.14,17.18,c:28,t:24,(-8.83,-2.47,;-7.5,-1.7,;-6.16,-.93,;-4.83,-.16,;-4.83,1.38,;-3.49,2.15,;-2.16,1.38,;-2.16,-.16,;-3.49,-.93,;-.83,-.93,;-.83,-2.47,;.51,-3.24,;1.84,-2.47,;3.3,-2.95,;4.21,-1.7,;4.98,-.37,;6.52,-.37,;7.29,-1.7,;8.83,-1.7,;6.52,-3.04,;4.98,-3.04,;3.3,-.46,;2.06,.45,;2.53,1.91,;1.76,3.24,;4.07,1.91,;4.84,3.24,;4.55,.45,;1.84,-.93,;.51,-.16,)| | ||
| Structure | 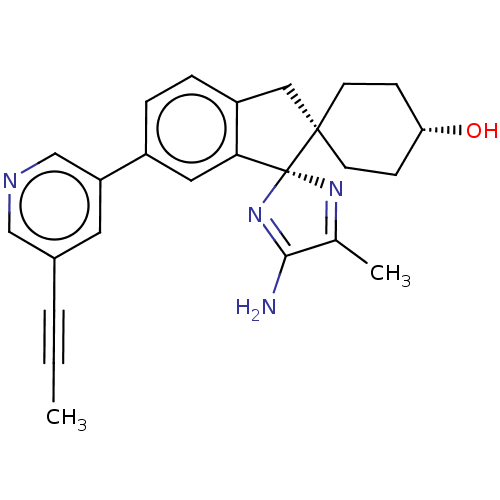
| ||