

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Protein Nef | ||
| Ligand | BDBM288052 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Measurement of Antiviral (HIV-1) Activity | ||
| Temperature | 298.15±n/a K | ||
| EC50 | 23.0±n/a nM | ||
| Comments | extracted | ||
| Citation |  Miyazaki, S; Isoshima, H; Oshita, K; Kawashita, S; Nagahashi, N; Terashita, M Substituted spiropyrido[1,2-a]pyrazine derivative and medicinal use thereof as HIV integrase inhibitor US Patent US10087178 Publication Date 10/2/2018 Miyazaki, S; Isoshima, H; Oshita, K; Kawashita, S; Nagahashi, N; Terashita, M Substituted spiropyrido[1,2-a]pyrazine derivative and medicinal use thereof as HIV integrase inhibitor US Patent US10087178 Publication Date 10/2/2018 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Protein Nef | |||
| Name: | Protein Nef | ||
| Synonyms: | 3ORF | F-protein | HIV-1 Nef | NEF_HV1BR | Negative factor | Protein Nef | nef | ||
| Type: | C-terminal core protein | ||
| Mol. Mass.: | 23340.47 | ||
| Organism: | Human immunodeficiency virus type 1 (isolate BRU/LAI group M subtype B) | ||
| Description: | P03406 | ||
| Residue: | 206 | ||
| Sequence: |
| ||
| BDBM288052 | |||
| n/a | |||
| Name | BDBM288052 | ||
| Synonyms: | N-(2,4-difluorobenzyl)-cis- 3,9'-dihydroxy-2'-methyl- 1',8'-dioxo-1',2',3',8'- tetrahydrospiro[cyclobutane- 1,4'-pyrido[1,2-a]pyrazine]- 7'-carboxamide hydrobromide | US10087178, Example S12 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H19F2N3O5 | ||
| Mol. Mass. | 419.3788 | ||
| SMILES | CN1C[C@]2(C[C@H](O)C2)n2cc(C(=O)NCc3ccc(F)cc3F)c(=O)c(O)c2C1=O |r,wU:3.7,wD:5.5,(8,2.63,;6.67,1.86,;6.67,.32,;5.33,-.45,;6.42,-1.54,;5.33,-2.63,;5.33,-4.17,;4.25,-1.54,;4,.32,;2.67,-.45,;1.33,.32,;,-.45,;,-1.99,;-1.33,.32,;-2.67,-.45,;-4,.32,;-4,1.86,;-5.33,2.63,;-6.67,1.86,;-8,2.63,;-6.67,.32,;-5.33,-.45,;-5.33,-1.99,;1.33,1.86,;,2.63,;2.67,2.63,;2.67,4.17,;4,1.86,;5.33,2.63,;5.33,4.17,)| | ||
| Structure | 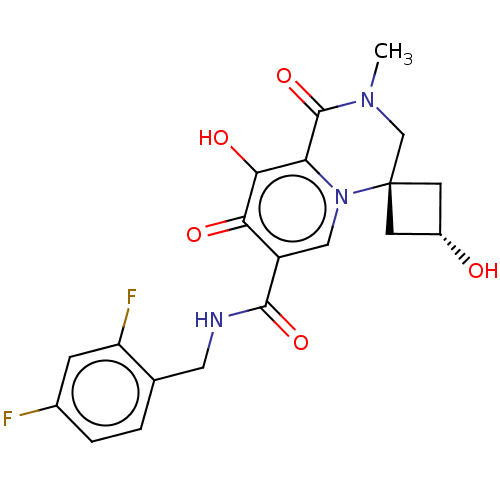
| ||