| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM189364 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | AChE Inhibitory Assay |
|---|
| pH | 7.7±n/a |
|---|
| IC50 | 83±3.2 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Shaik, JB; Palaka, BK; Penumala, M; Eadlapalli, S; Darla Mark, M; Ampasala, DR; Vadde, R; Amooru Gangaiah, D Synthesis, Biological Evaluation, and Molecular Docking of 8-imino-2-oxo-2H,8H-pyrano[2,3-f]chromene Analogs: New Dual AChE Inhibitors as Potential Drugs for the Treatment of Alzheimer's Disease. Chem Biol Drug Des88:43-53 (2016) [PubMed] Article Shaik, JB; Palaka, BK; Penumala, M; Eadlapalli, S; Darla Mark, M; Ampasala, DR; Vadde, R; Amooru Gangaiah, D Synthesis, Biological Evaluation, and Molecular Docking of 8-imino-2-oxo-2H,8H-pyrano[2,3-f]chromene Analogs: New Dual AChE Inhibitors as Potential Drugs for the Treatment of Alzheimer's Disease. Chem Biol Drug Des88:43-53 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_ELEEL | Acetylcholinesterase (AChE) | Acetylcholinesterase (EeAChE) | ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 71812.79 |
|---|
| Organism: | Electrophorus electricus (Electric eel) |
|---|
| Description: | n/a |
|---|
| Residue: | 633 |
|---|
| Sequence: | MKILDALLFPVIFIMFFIHLSIAQTDPELTIMTRLGQVQGTRLPVPDRSHVIAFLGIPFA
EPPLGKMRFKPPEPKKPWNDVFDARDYPSACYQYVDTSYPGFSGTEMWNPNRMMSEDCLY
LNVWVPATPRPHNLTVMVWIYGGGFYSGSSSLDVYDGRYLAHSEKVVVVSMNYRVSAFGF
LALNGSAEAPGNVGLLDQRLALQWVQDNIHFFGGNPKQVTIFGESAGAASVGMHLLSPDS
RPKFTRAILQSGVPNGPWRTVSFDEARRRAIKLGRLVGCPDGNDTDLIDCLRSKQPQDLI
DQEWLVLPFSGLFRFSFVPVIDGVVFPDTPEAMLNSGNFKDTQILLGVNQNEGSYFLIYG
APGFSKDNESLITREDFLQGVKMSVPHANEIGLEAVILQYTDWMDEDNPIKNREAMDDIV
GDHNVVCPLQHFAKMYAQYSILQGQTGTASQGNLGWGNSGSASNSGNSQVSVYLYMFDHR
ASNLVWPEWMGVIHGYEIEFVFGLPLEKRLNYTLEEEKLSRRMMKYWANFARTGNPNINV
DGSIDSRRRWPVFTSTEQKHVGLNTDSLKVHKGLKSQFCALWNRFLPRLLNVTENIDDAE
RQWKAEFHRWSSYMMHWKNQFDHYSKQERCTNL
|
|
|
|---|
| BDBM189364 |
|---|
| n/a |
|---|
| Name | BDBM189364 |
|---|
| Synonyms: | N-dodecyl-8-imino-2-oxo-2H,8H-pyrano[2,3-f]chromene-9-carboxamide (4c) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H32N2O4 |
|---|
| Mol. Mass. | 424.5326 |
|---|
| SMILES | CCCCCCCCCCCCNC(=O)c1cc2c(ccc3ccc(=O)oc23)oc1=N |
|---|
| Structure | 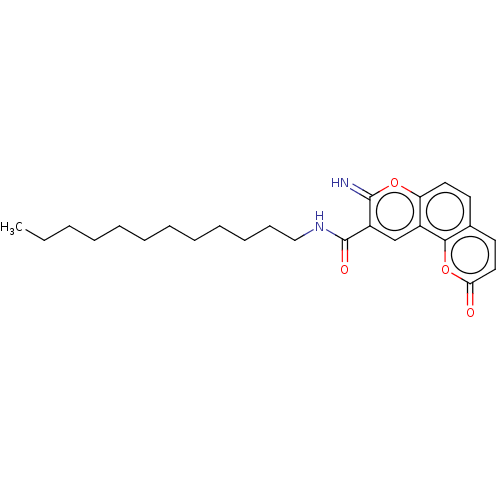
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shaik, JB; Palaka, BK; Penumala, M; Eadlapalli, S; Darla Mark, M; Ampasala, DR; Vadde, R; Amooru Gangaiah, D Synthesis, Biological Evaluation, and Molecular Docking of 8-imino-2-oxo-2H,8H-pyrano[2,3-f]chromene Analogs: New Dual AChE Inhibitors as Potential Drugs for the Treatment of Alzheimer's Disease. Chem Biol Drug Des88:43-53 (2016) [PubMed] Article
Shaik, JB; Palaka, BK; Penumala, M; Eadlapalli, S; Darla Mark, M; Ampasala, DR; Vadde, R; Amooru Gangaiah, D Synthesis, Biological Evaluation, and Molecular Docking of 8-imino-2-oxo-2H,8H-pyrano[2,3-f]chromene Analogs: New Dual AChE Inhibitors as Potential Drugs for the Treatment of Alzheimer's Disease. Chem Biol Drug Des88:43-53 (2016) [PubMed] Article