| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase |
|---|
| Ligand | BDBM201512 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | MGAT LCMS Assay |
|---|
| pH | 7.4±n/a |
|---|
| IC50 | 2±n/a nM |
|---|
| Comments | extracted |
|---|
| Citation |  Turdi, H; Hangeland, JJ; Lawrence, RM; Cheng, D; Ahmad, S; Meng, W; Brigance, RP; Devasthale, P; Zhao, G Aryl dihydropyridinones and piperidinone MGAT2 inhibitors US Patent US9187424 Publication Date 11/17/2015 Turdi, H; Hangeland, JJ; Lawrence, RM; Cheng, D; Ahmad, S; Meng, W; Brigance, RP; Devasthale, P; Zhao, G Aryl dihydropyridinones and piperidinone MGAT2 inhibitors US Patent US9187424 Publication Date 11/17/2015 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase |
|---|
| Name: | Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase |
|---|
| Synonyms: | 2.4.1.143 | Beta-1,2-N-acetylglucosaminyltransferase II | GNT-II | GlcNAc-T II | MGAT2 | MGAT2_HUMAN | Mannoside acetylglucosaminyltransferase 2 | Monoacylglycerol acyltransferase 2 (MGAT2) | Monoacylglycerol acyltransferase type 2 (h-MGAT2) | N-glycosyl-oligosaccharide-glycoprotein N-acetylglucosaminyltransferase II | h-MGAT2 (human monoacylglycerol acyltransferase type 2) |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 51567.80 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q10469 |
|---|
| Residue: | 447 |
|---|
| Sequence: | MRFRIYKRKVLILTLVVAACGFVLWSSNGRQRKNEALAPPLLDAEPARGAGGRGGDHPSV
AVGIRRVSNVSAASLVPAVPQPEADNLTLRYRSLVYQLNFDQTLRNVDKAGTWAPRELVL
VVQVHNRPEYLRLLLDSLRKAQGIDNVLVIFSHDFWSTEINQLIAGVNFCPVLQVFFPFS
IQLYPNEFPGSDPRDCPRDLPKNAALKLGCINAEYPDSFGHYREAKFSQTKHHWWWKLHF
VWERVKILRDYAGLILFLEEDHYLAPDFYHVFKKMWKLKQQECPECDVLSLGTYSASRSF
YGMADKVDVKTWKSTEHNMGLALTRNAYQKLIECTDTFCTYDDYNWDWTLQYLTVSCLPK
FWKVLVPQIPRIFHAGDCGMHHKKTCRPSTQSAQIESLLNNNKQYMFPETLTISEKFTVV
AISPPRKNGGWGDIRDHELCKSYRRLQ
|
|
|
|---|
| BDBM201512 |
|---|
| n/a |
|---|
| Name | BDBM201512 |
|---|
| Synonyms: | US9187424, 279 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H27F3N2O5S |
|---|
| Mol. Mass. | 572.595 |
|---|
| SMILES | Cc1ccc(cc1)C1=C(NC(=O)CS(C)(=O)=O)C(=O)NC(C1)(c1ccc(OCc2ccccc2)cc1)C(F)(F)F |c:8| |
|---|
| Structure | 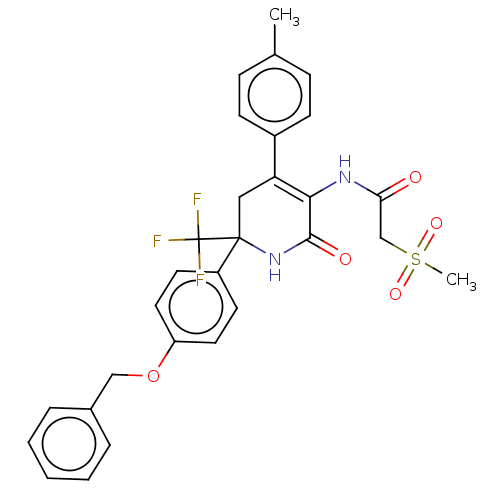
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Turdi, H; Hangeland, JJ; Lawrence, RM; Cheng, D; Ahmad, S; Meng, W; Brigance, RP; Devasthale, P; Zhao, G Aryl dihydropyridinones and piperidinone MGAT2 inhibitors US Patent US9187424 Publication Date 11/17/2015
Turdi, H; Hangeland, JJ; Lawrence, RM; Cheng, D; Ahmad, S; Meng, W; Brigance, RP; Devasthale, P; Zhao, G Aryl dihydropyridinones and piperidinone MGAT2 inhibitors US Patent US9187424 Publication Date 11/17/2015